My take on the dugout loop. Yes, around the end I mistyped “lifestyle”, I’m very sorry, oh wait, I’m not.
Check out my Patreon tiers! https://www.patreon.com/adamsomething
My take on the dugout loop. Yes, around the end I mistyped “lifestyle”, I’m very sorry, oh wait, I’m not.
Check out my Patreon tiers! https://www.patreon.com/adamsomething
Engineers have designed a computer processor that thwarts hackers by randomly changing its microarchitecture every few milliseconds. Known as Morpheus, the puzzling processor has now aced its first major tests, repelling hundreds of professional hackers in a DARPA security challenge.
In 2017, DARPA backed the University of Michigan’s Morpheus project with US$3.6 million in funding, and now the novel processor has been put to the test. Over four months in 2020, DARPA ran a bug bounty program called Finding Exploits to Thwart Tampering (FETT), pitting 525 professional security researchers against Morpheus and a range of other processors.
The goal of the program was to test new hardware-based security systems, which could protect data no matter how vulnerable the underlying software was. Morpheus was mocked up to resemble a medical database, complete with software vulnerabilities – and yet, not a single attack made it through its defenses.


In 2019, the European Union banned chlorpyrifos, allowing three more months of use. Canada’s three-year phaseout risks ongoing harms, as well as dumping of this product on our market.
Back in 2000, Canadian politicians spoke up against commonly used lawn and household chlorpyrifos products when the U.S. banned domestic uses. Despite a year of study, the PMRA had not taken action.
How long will chlorpyrifos persist in commerce?

Circa 2010
Medical researchers use laboratory-grown human cells to learn the intricacies of how cells work and test theories about the causes and treatment of diseases. The cell lines they need are “immortal”—they can grow indefinitely, be frozen for decades, divided into different batches and shared among scientists. In 1951, a scientist at Johns Hopkins Hospital in Baltimore, Maryland, created the first immortal human cell line with a tissue sample taken from a young black woman with cervical cancer. Those cells, called HeLa cells, quickly became invaluable to medical research—though their donor remained a mystery for decades. In her new book, The Immortal Life of Henrietta Lacks, journalist Rebecca Skloot tracks down the story of the source of the amazing HeLa cells, Henrietta Lacks, and documents the cell line’s impact on both modern medicine and the Lacks family.
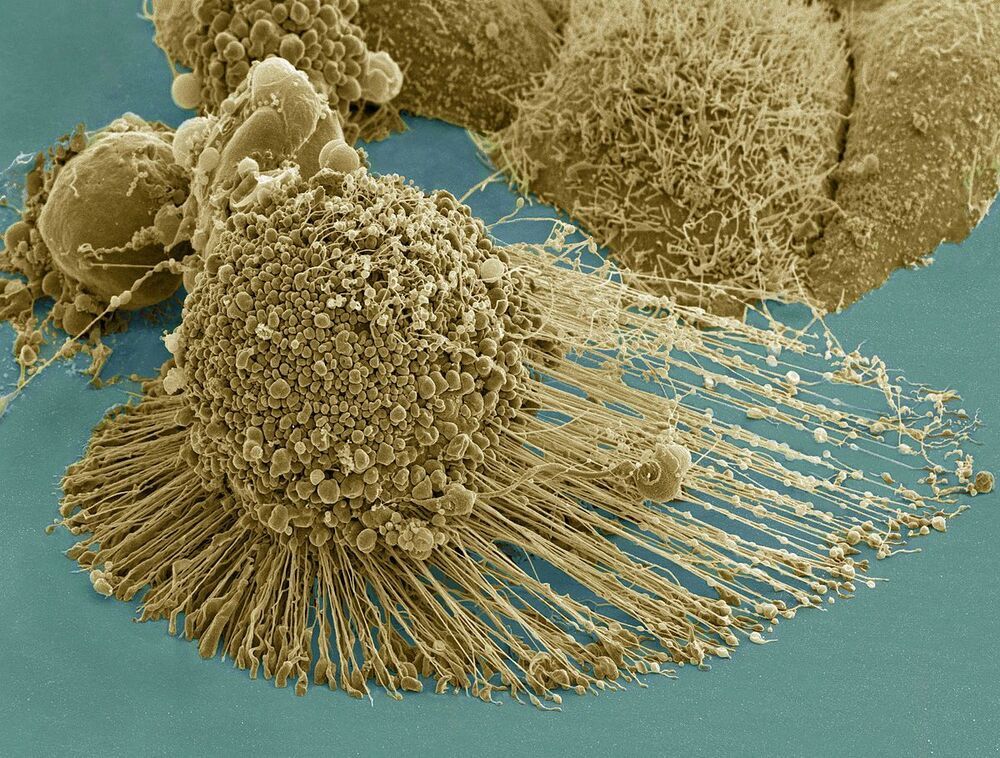
HeLa (/ ˈ h iː l ɑː / ; also Hela or hela) is an immortal cell line used in scientific research. It is the oldest and most commonly used human cell line.[1] The line is named after and derived from cervical cancer cells taken on February 8, 1951,[2] from Henrietta Lacks, a 31-year-old African-American mother of five, who died of cancer on October 4, 1951.[3] The cell line was found to be remarkably durable and prolific, which allows it to be used extensively in scientific study.[4][5]
Scanning electron micrograph of an apoptotic HeLa cell. Zeiss Merlin HR-SEM.


Fireflies.ai, an AI-powered videoconference note-taking tool, has raised $14 million in a series A funding round.
Some might like.
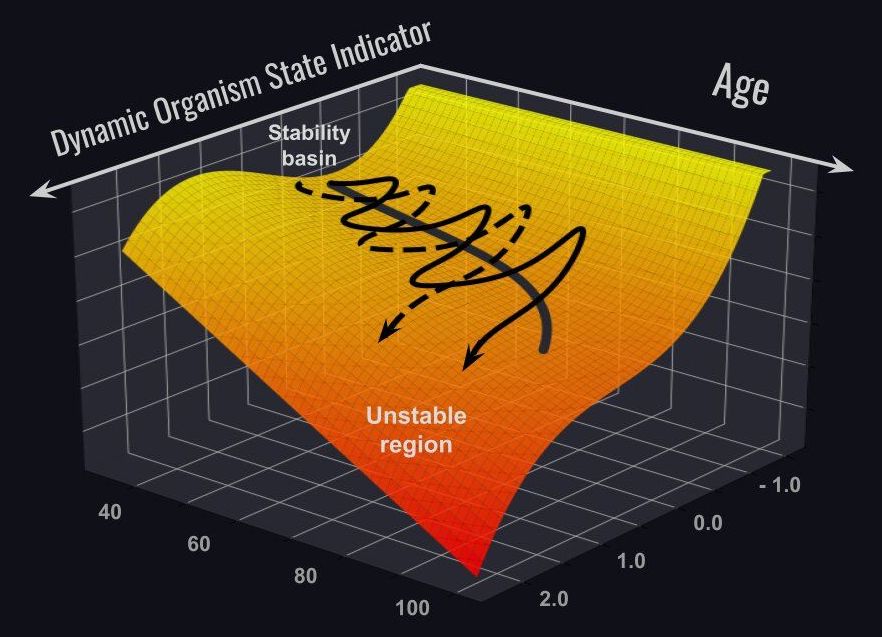
The research team of Gero, a Singapore-based biotech company in collaboration with Roswell Park Comprehensive Cancer Center in Buffalo NY, has presented a study in Nature Communications on associations between aging and the loss of the ability to recover from stresses.
Recently, scientists have reported the first promising examples of biological age reversal by experimental interventions. Indeed, many biological clock types properly predict more years of life for those who choose healthy lifestyles or quit unhealthy ones, such as smoking. Still unknown is how quickly biological age is changing over time for the same individual, and distinguishing between the transient fluctuations and the genuine bioage change trend.
The emergence of big biomedical data involving multiple measurements from the same subjects brings about a whole range of novel opportunities and practical tools to understand and quantify the aging process in humans. A team of experts in biology and biophysics presented results of a detailed analysis of dynamic properties of the fluctuations of physiological indices along individual aging trajectories.

Might interest some.
A new study from the Institute of Psychiatry, Psychology and Neuroscience (IoPPN) at King’s College London has established that Intermittent Fasting (IF) is an effective means of improving long term memory retention and generating new adult hippocampal neurons in mice, in what the researchers hope has the potential to slow the advance of cognitive decline in older people.
The study, published today in Molecular Biology, found that a calorie restricted diet via every other day fasting was an effective means of promoting Klotho gene expression in mice. Klotho, which is often referred to as the “longevity gene” has now been shown in this study to play a central role in the production of hippocampal adult-born new neurons or neurogenesis.
Adult-born hippocampal neurons are important for memory formation and their production declines with age, explaining in part cognitive decline in older people.