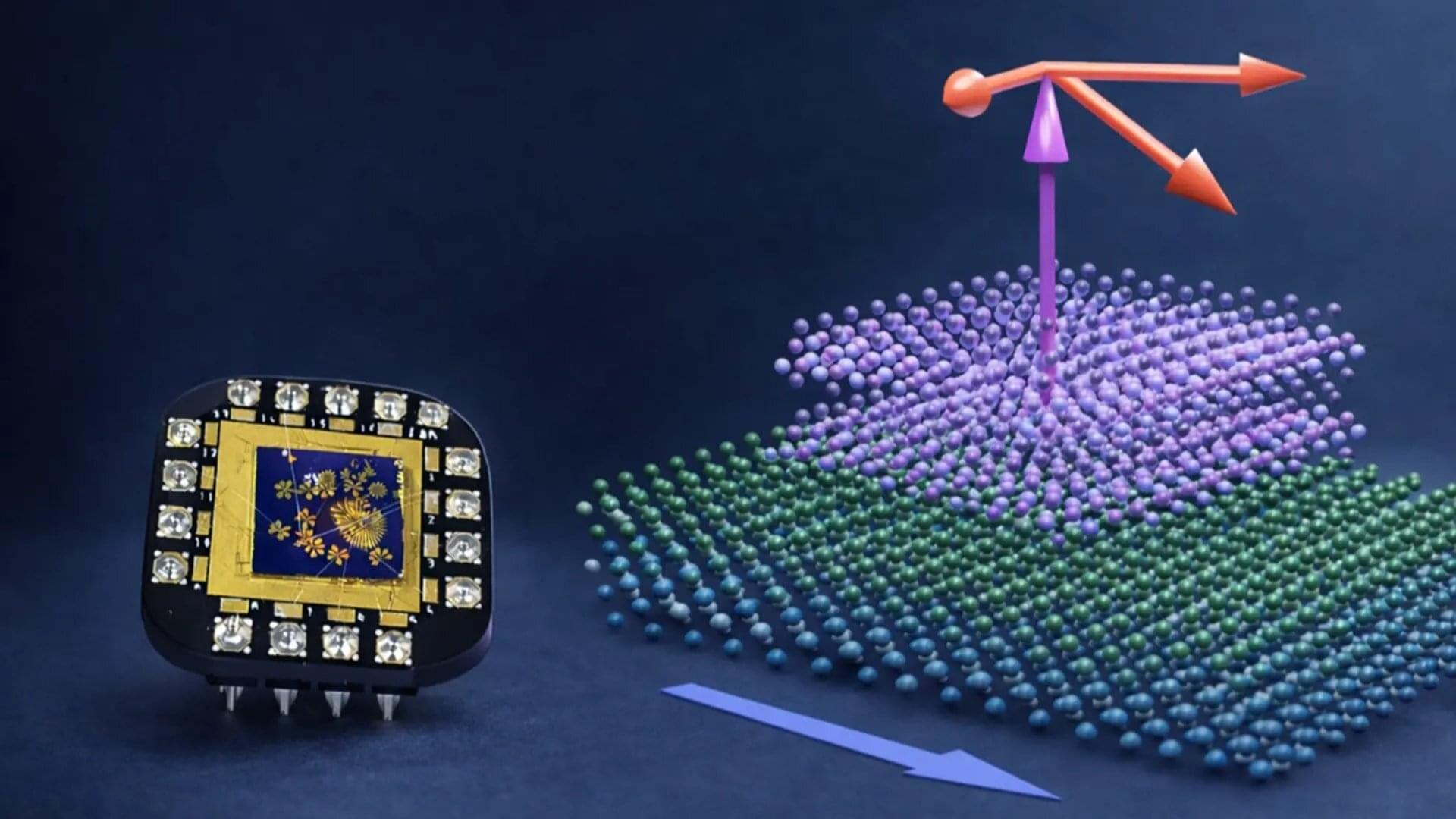
Multi-titanium hydrides can selectively snip the strong structural bonds of stable organic molecules called pyridines, RIKEN researchers have shown. This discovery could guide designing catalysts for applications in multiple branches of industrial chemistry, from oil refining to the synthesis of functional organic molecules. The findings are published in the Journal of the American Chemical Society.
Pyridines are stable aromatic molecules characterized by a ring consisting of one nitrogen atom and five carbon atoms. They are a common structural motif in complex organic molecules such as pharmaceuticals. They are also a component of crude oil that needs to be removed during refining.
“The removal of nitrogen-containing impurities such as pyridines from crude oil is an important industrial process in petroleum refining,” notes Zhaomin Hou of the RIKEN Organometallic Chemistry Laboratory and the RIKEN Advanced Catalysis Research Group.