Physicists recently mapped the hidden shape that underlies the quantum behaviors of a crystal, using a new method that’s expected to become ubiquitous.



A research team led by Prof. Seunguk Song from the Department of Energy Science at Sungkyunkwan University (SKKU), in collaboration with the Institute for Basic Science (IBS), the University of Pennsylvania, and the U.S. Air Force Research Laboratory, has developed a comprehensive technical roadmap for two-dimensional (2D) indium selenides (InSe)—a key material for next-generation low-power and quantum computing.
The study, titled “Indium selenides for next-generation electronics and optoelectronics,” was published in Nature Reviews Electrical Engineering. This research provides a deep dive into the physical properties and device applications of 2D quantum semiconductors, which are viewed as a definitive alternative to silicon as it reaches its physical scaling limits.
As current silicon-based semiconductors shrink to the sub-nanometer scale, they face critical hurdles such as surging power consumption, overheating, and leakage current. To address these challenges, Professor Song’s team focused on InSe, an atomically thin material.

https://youtube.com/@FrontierofScience?sub_confirmation=1
👉Join my channel for exclusive monthly recap videos and help support the project: https://www.youtube.com/channel/UCFedj48BfqrEhke2Na7UK7w/join.
What if void creates matter? this is no longer a philosophical question but an experimental reality. A landmark study published in Nature by the STAR collaboration at the Relativistic Heavy Ion Collider of Brookhaven National Laboratory has, for the first time in history, directly observed virtual particles emerging from the quantum vacuum and becoming real matter. By colliding protons at 99% of the speed of light, scientists excited the quantum vacuum and tracked the precise moment transient quark-antiquark pairs materialized into measurable physical entities.
The experiment revealed something even more profound: particle pairs born from the void carry a measurable spin alignment, a direct signature of quantum entanglement inherited from the vacuum’s chiral condensate. This correlation had no other conceivable explanation than the particles having truly emerged from nothing. The implications extend far beyond particle physics: nearly 99% of the mass of everything that exists, including our own bodies, derives not from the Higgs mechanism, but from the incessant interaction between real quarks and the swarm of virtual particles that populate the quantum vacuum.
what if void creates matter reframes our understanding of reality at its deepest level. The boundary between being and non-being dissolves, revealing that “nothing” is an extraordinarily dense and generative condition. Quantum mechanics remains our most precise but still incomplete map of the universe, yet discoveries like this bring us closer to grasping a cosmos that, starting from the vacuum, generates the infinite.
#quantumvacuum #vacum #science #quantumphysics #entanglement #quantumentanglement #quantumgravity #gravity #generalrelativity #quantummechanics #quantumconsciousness #quantum #quantumweirdness #materialism #awareness #consciuosness #hardproblem #einstein #time #timeisanillusion #retrocausality #doubleslitexperiment #penrose #rogerpenrose #multiverse #manyworlds #paralleluniverse.
TIMESTAMPS
00:00 Introduction: What If Void Creates Matter.
01:16 Heisenberg’s Uncertainty Principle and Quantum Vacuum Fluctuations.
02:12 Virtual Particles and the Casimir Effect.
02:52 The STAR Collaboration Study Published in Nature.
03:27 The Brookhaven Experiment: Exciting the Quantum Vacuum.
04:16 Quantum Entanglement Born Directly from the Void.
05:03 Lambda Hyperons and the Proof of Materialization.
06:10 What It Means That Matter Emerges from Nothing.
06:22 What If Void Creates Matter: The True Origin of Mass.
07:33 Philosophical Implications: Reality, Time, and the Nature of Existence.
⚠️ This video is entirely written, edited, and produced by me in an original way. For practical reasons, I used a synthetic voice, but nothing is automated: every concept comes from my dedication, my research, and a profound passion for science.
In this video, we explore seven of the most profound and unsettling theories in modern cosmology. From Conformal Cyclic Cosmology, where the death of a previous universe becomes our beginning, to the idea that our entire cosmos emerged from a quantum fluctuation out of “nothing.” We dive into Loop Quantum Cosmology and the Big Bounce, brane collisions in higher dimensions, eternal inflation creating infinite bubble universes, a CPT-symmetric mirror anti-universe flowing backward in time, and finally the terrifying scale of the String Landscape and the multiverse.
These theories challenge everything we think we know about reality, time, and existence itself. If even one of them is correct, the Big Bang wasn’t the beginning — it was just one event in something far larger, stranger, and possibly eternal.
The universe may not have started. It may have restarted.
Subscribe for more deep space explorations.
#BigBang #Cosmology #Multiverse

Superconductivity is a quantum state of matter characterized by an electrical resistance of zero and the expulsion of magnetic fields at low temperatures below a critical point. Superconductors, materials in which this state occurs, have proved to be highly advantageous for the development of various technologies, including medical imaging devices, particle accelerators and quantum computers.
While superconductivity typically only occurs at extremely low temperatures, recent studies showed that in some materials it can arise at higher temperatures. These unconventional superconducting materials are referred to as high-temperature (high-Tc) superconductors.
Researchers at the National Laboratory of Solid-State Microstructures and Nanjing University recently gathered hints of high-Tc superconductivity in a thin film nickelate, a material that contains nickel and oxygen arranged in a thin layered crystal structure. Their paper, published in Physical Review Letters, maps the evolution of physical states in these materials under different conditions, unveiling a so-called “superconducting dome” in this phase diagram, which is associated with high-Tc superconductivity.

A new CRISPR-powered light sensor can detect the faintest molecular signs of cancer in a drop of blood. A new light-based sensor can spot incredibly tiny amounts of cancer biomarkers in blood, raising the possibility of earlier and simpler cancer detection. The technology merges DNAnanotechnology, CRISPR, and quantumdots to generate a clear signal from just a few molecules. In lung cancer tests, it worked even in real patient serum samples. Researchers hope it could eventually power portable blood tests for cancer and other diseases.
Scientists have designed a powerful light based sensor capable of detecting extremely small amounts of cancer biomarkers in blood. The innovation could eventually allow doctors to identify early warning signs of cancer and other diseases through a routine blood draw.
Biomarkers such as proteins, fragments of DNA, and other molecules can signal whether cancer is present, how it is progressing, or a person’s risk of developing it. The difficulty is that in the earliest stages of disease, these markers exist in extremely low concentrations, making them hard to measure with conventional tools.

Daily reflection is a way to apply this principle in our everyday lives. It shines a spotlight on the behavior itself. And when behavior is observed consistently, it solidifies into neural pathways in the brain. We start behaving differently, not because someone else is judging us, but because we are measuring ourselves. The simple act of asking ourselves reflective questions each day shapes the behaviors in our lives, which, in turn, make us the people who exhibit those behaviors.
Another principle from quantum theory, entanglement, might also be at play when we do daily reflection. Quantum entanglement describes how particles can become linked to one another so that a change in one results in a change in the other. In the same way, the effort we make to change in one part of our lives is rarely confined to that part. Instead, our behaviors extend outward and affect those in relationship to us and around us. For example, your attempt to speak in positive terms, rather than negative ones, can influence your colleagues at work. Your intention to control your emotional outbursts can affect your family. Your efforts to build positive relationships at work or in your community can change the dynamics of those relationships. And when you combine these intentions with daily reflection, you’re not only strengthening a positive personal trait within yourself, but also influencing the bigger, interpersonal systems around you.
Philosophers, physicians, and physicists are forever debating what consciousness is. Is who we are just a byproduct of biology and the brain’s physiology, or is who we are more fundamental and exists irrespective of the brain’s neural firing? We may never know. That said, one thing is true: Conscious awareness shapes who we are. Without reflection, behavior defaults to habit. With reflection, possibility re-enters the system. The practice of asking yourself daily reflective questions puts you in the role of an observer rather than an actor. And from there, you can be intentional about who you choose to be tomorrow.

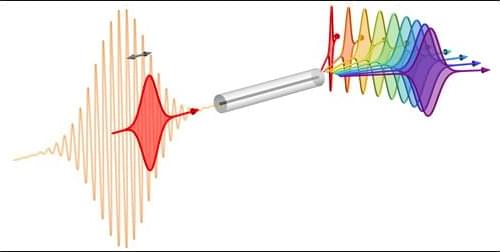
Researchers can adjust the frequency and bandwidth of single photons inside an optical fiber, which will be useful for future quantum networks.
Future quantum technologies will require practical techniques for adjusting the frequencies and bandwidths of individual photons to optimize them for various purposes without losing the delicate quantum data that they carry. Now researchers have improved on previous technology and have shown how both properties can be tuned over a wide range inside a short length of standard optical fiber [1]. They expect that this technique will be more practical and effective than current alternatives and will find wide use in interfacing devices in future quantum computing and communications networks.
Photons are likely to provide the means for transmitting information within future quantum networks, but frequent changes to their properties will be required in order for them to carry out a diversity of tasks. For example, a trapped-ion quantum memory emits or absorbs photons at a specific visible wavelength with an extremely narrow bandwidth, which means that a photon with which it interacts must be produced as a relatively long light pulse. In contrast, a high-speed fiber-optic channel works best with infrared photons having much broader bandwidths, which require short light pulses.