Researchers have made a significant advance toward the goal of using bacteria to produce ethylene, a key manufacturing chemical.




SereNeuro Therapeutics, a preclinical biotechnology company developing non-opioid pain therapies, has unveiled new data on a novel approach to chronic pain management and joint tissue preservation. The data highlight SN101, a first-in-class induced pluripotent stem cell (iPSC)-derived therapy.
The announcement was made at the International Society for Stem Cell Research (ISSCR) Symposium Accelerating PSC-Derived Cell Therapies: Starting with the End in Mind.

Today, leading AI technology such as large language models (LLMs) have begun to transform how we access and work with abstract knowledge. Yet they remain wordsmiths in the dark, eloquent but inexperienced, knowledgeable but ungrounded.
For humans, spatial intelligence is the scaffolding upon which our cognition is built. It’s at work when we passively observe or actively seek to create. It drives our reasoning and planning, even on the most abstract topics. And it’s essential to the way we interact—verbally or physically, with our peers or with the environment itself. When machines are endowed with this ability, it will transform how we create and interact with real and virtual worlds—revolutionizing storytelling, robotics, scientific discovery, and beyond. This is AI’s next frontier, and why 2025 was such a pivotal year.
The candid truth is that AI’s spatial capabilities remain far from the human level. But tremendous progress has indeed been made. Multimodal LLMs, trained with voluminous multimedia data in addition to textual data, have introduced some basics of spatial awareness, and today’s AI can analyze pictures, answer questions about them, and generate hyperrealistic images and short videos.
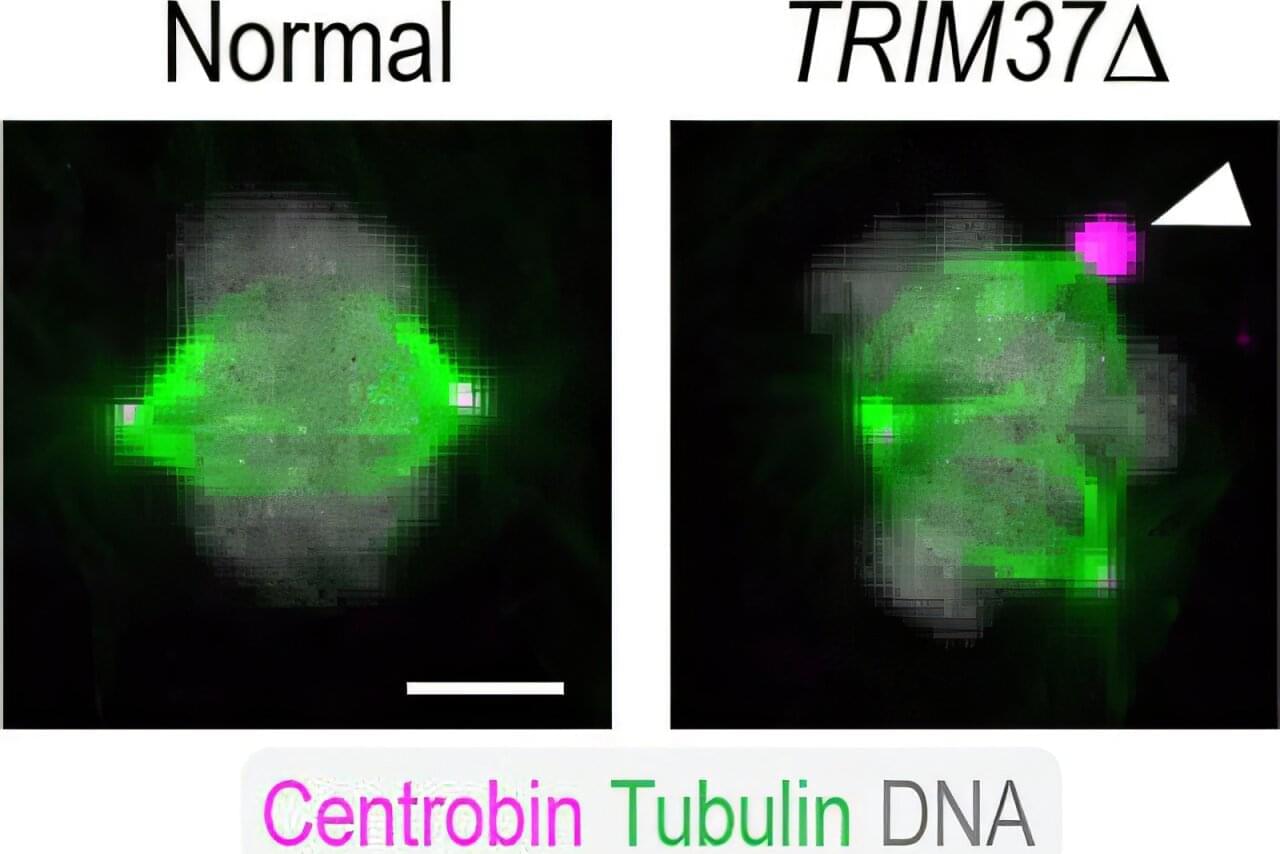
In 2000, researchers discovered that mutations that inactivate a gene known as TRIM37 cause a developmental disease called Mulibrey nanism. The extremely rare inherited disorder leads to growth delays and abnormalities in several organs, causing afflictions of the heart, muscles, liver, brain and eyes. In addition, Mulibrey nanism patients exhibit high rates of cancer and are infertile.
In 2016, UC San Diego School of Biological Sciences researchers in the labs of Professors Karen Oegema and Arshad Desai began understanding how TRIM37, when operating normally, plays a key role in preventing conditions that lead to Mulibrey nanism. They linked TRIM37 to spindles, which separate chromosomes during cell division, and centrosomes, the spherical organizing structures at each end of spindles.
The image above shows a normal mitotic cell (left) compared to a cell lacking TRIM37 (right), with spindle microtubules (green), centrosomal protein centrobin (magenta) and DNA (white). Normal cells have two spindle poles that ensure proper cell division. Cells lacking TRIM37 frequently have extra spindle poles, containing a cluster of centrobin molecules that disrupt proper cell division. Patients with Mulibrey nanism lack TRIM37 and their cells show similar extra spindle poles.


There is still relatively little known about the genetic underpinnings of proteomic aging clocks. Here, we describe a genome-wide association study of proteomic aging in the UK Biobank (n=38,865), identifying 27 loci associated with participants’ proteomic age gap (ProtAgeGap). ProtAgeGap exhibits a strong genetic correlation with longevity (rg = −0.83), and in FinnGen a ProtAgeGap polygenic score (PGS) was associated with significantly increased odds of achieving longevity (n=500,348; OR = 1.43). Additional PGS analyses in All of Us (n=117,415), China Kadoorie Biobank (n=100,640), and ABCD Study (n=5,204) demonstrate reproducible associations across biobanks of ProtAgeGap PGS with obesity, cardiometabolic disease, and osteoarthritis in adults, and with developmental timing in children. Finally, colocalization analysis identified FTO as an obesity-related mechanism uniting diverse aging traits. Our results demonstrate a shared genetic architecture across the life course of ProtAgeGap with longevity, early developmental biology, and cardiometabolic and musculoskeletal diseases.
### Competing Interest Statement.
The authors have declared no competing interest.
For much of my career, I have been fascinated by the ways in which materials behave when we reduce their dimensions to the nanoscale. Over and over, I’ve learned that when we shrink a material down to just a few nanometers in thickness, the familiar textbook rules of physics begin to bend, stretch, or sometimes break entirely. Heat transport is one of the areas where this becomes especially intriguing, because heat is carried by phonons—quantized vibrations of the atomic lattice—and phonons are exquisitely sensitive to spatial confinement.
A few years ago, something puzzling emerged in the literature. Molecular dynamics simulations showed that ultrathin silicon films exhibit a distinct minimum in their thermal conductivity at around one to two nanometers thickness, which corresponds to just a few atomic layers. Even more surprisingly, the thermal conductivity starts to increase again if the material is made even thinner, approaching extreme confinement and the 2D limit.
This runs counter to what every traditional model would predict. According to classical theories such as the Boltzmann transport equation or the Fuchs–Sondheimer boundary-scattering framework, reducing thickness should monotonically suppress thermal conductivity because there is simply less room for phonons to travel freely and carry heat around. Yet the simulations done by the team of Alan McGaughey at Carnegie Mellon University in Pittsburgh insisted otherwise, and no established theory could explain why.