Why do certain immune cells remain permanently active in allergic asthma – even in an environment that should actually damage them? A research team has discovered that these cells only survive because they activate a special antioxidant protection mechanism. When this mechanism is blocked, allergic inflammation in mouse models decreases significantly. The results have now been published in the scientific journal Immunity.
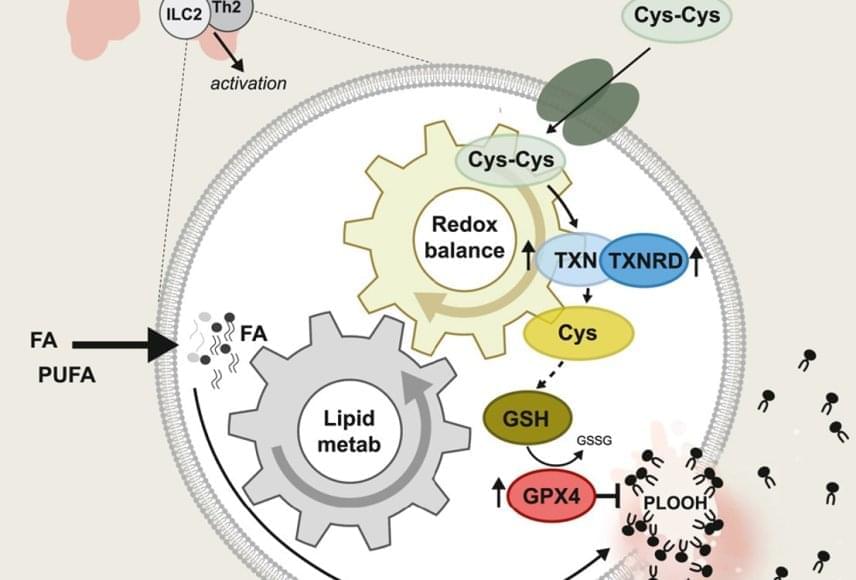
In allergic asthma, so-called ILC2 and Th2 cells are key drivers of inflammation. They produce messenger substances that increase mucus formation and the influx of immune cells. At the same time, the inflamed lung tissue is rich in free fatty acids and oxidative molecules — conditions that normally endanger cells.
The study shows that pathogenic ILC2s absorb large amounts of these fats and incorporate them into their membranes. In order to avoid dying from ferroptosis, an iron-dependent form of cell death caused by oxidized lipids, they activate their antioxidant systems. The enzymes GPX4 and TXNRD1 play a central role in this process. They neutralize harmful lipid peroxides and enable the cells to survive and multiply despite the stressful environment.
To test this approach, the Bonn team inhibited the thioredoxin metabolic pathway using a drug that blocks the enzyme TXNRD1. In mouse models, this led to significantly less ILC2 accumulating in the lungs. As a result, both the production of the typical type 2 cytokines IL-5 and IL-13 and the number of eosinophils and mucus production decreased. Overall, the allergic reaction was significantly less severe.