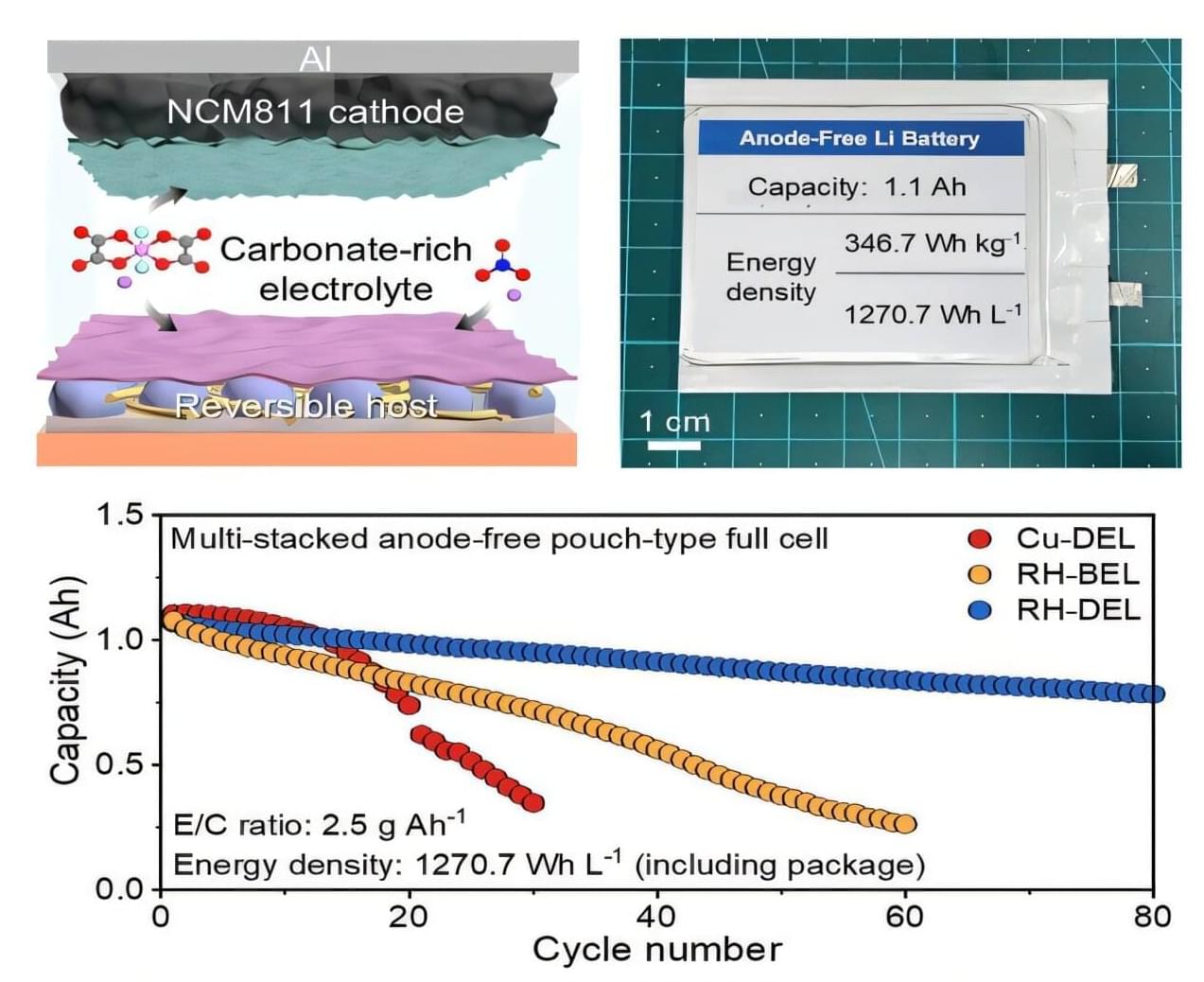
Bipolar disorder (BD) is a psychiatric disorder characterized by extreme mood changes. Individuals diagnosed with BD typically alternate between periods of high energy, euphoria, irritability and/or impulsivity (i.e., manic episodes) and others marked by feelings of sadness, low energy, and hopelessness (i.e., depression).
While there are now several medications that can help patients to manage the disorder and stabilize their mood, many of these drugs have side effects and dosages often need to be periodically adjusted. Recent studies suggest that the bacteria and microorganisms living in the digestive system, also known as gut microbiota, play a key role in mental health and might also contribute to some symptoms of BD.
Researchers at Zhejiang University, the Nanhu Brain-Computer Interface Institute and other institutes recently carried out a study investigating the possible connection between gut microbiota and the depressive episodes experienced by people diagnosed with BD. Their findings, published in Molecular Psychiatry, suggest that the microorganisms in the digestive system can directly influence connections between specific brain regions known to be affected by BD depression.