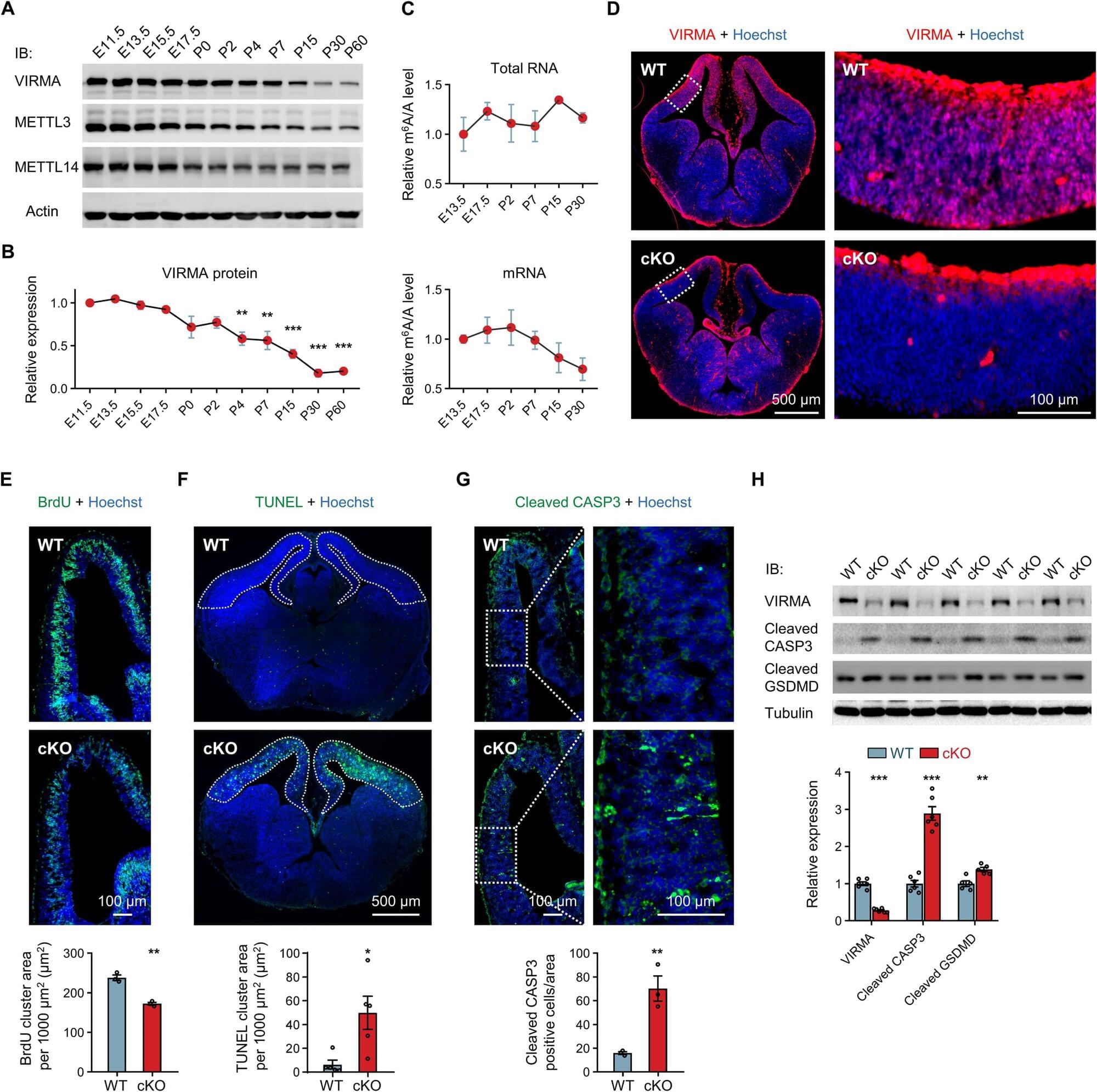
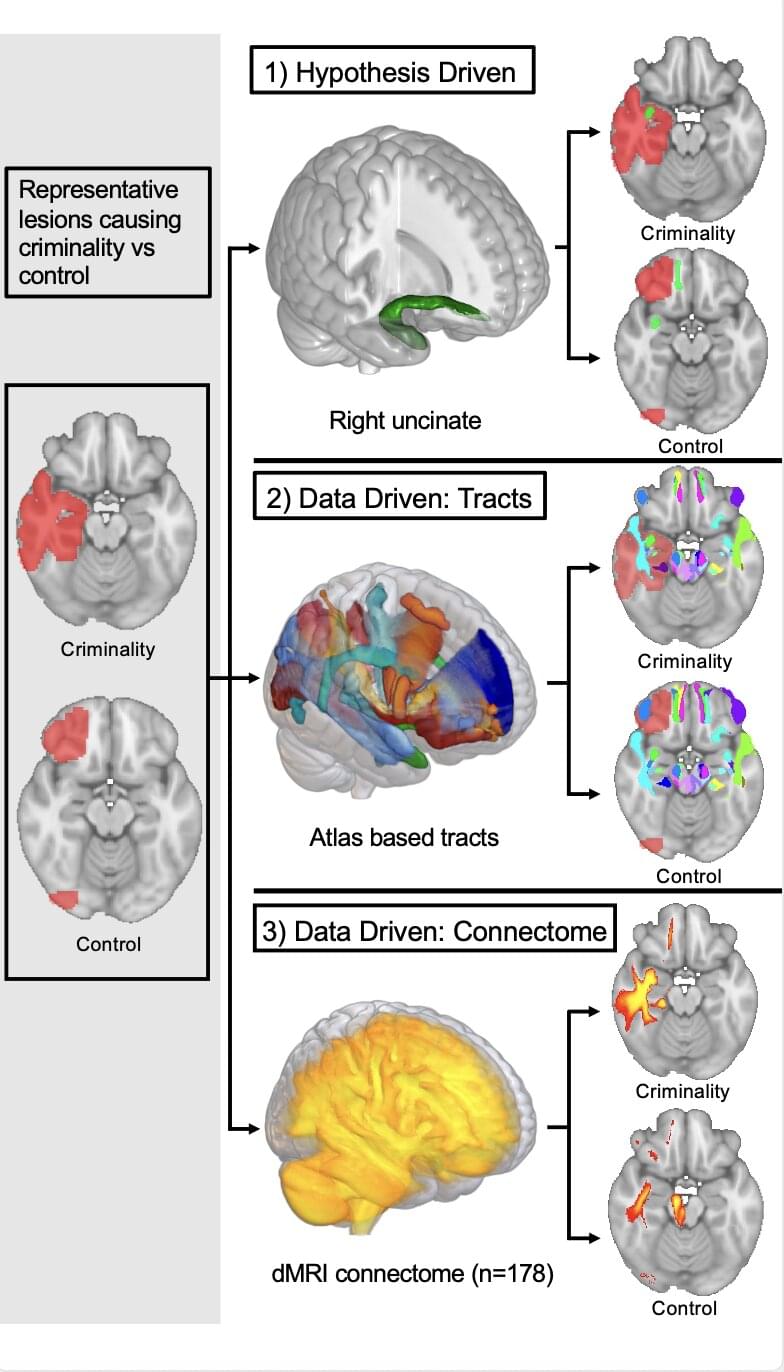
Our brain makes decisions based on direct associations between stimuli in our environment, but it often also does so based on events that initially appear unrelated. How does it achieve this? A recent study by the Cellular Mechanisms in Physiological and Pathological Behavior Research Group at the Hospital del Mar Research Institute, published in Proceedings of the National Academy of Sciences, offers new insights into this process and identifies the brain areas involved.
Using observations in mice, led primarily by first author and Ph.D. student José Antonio González Parra and supervised by Dr. Arnau Busquets, the research team was able to determine the mechanisms involved in how the brain makes decisions based on indirect associations between different stimuli. That is, instead of directly associating a specific stimulus with a rewarding or aversive situation, the brain establishes connections between two or more stimuli.
Dr. Busquets explains, “The project aims to understand how the brain enables us to make decisions based on indirect relationships between stimuli in our environment.”