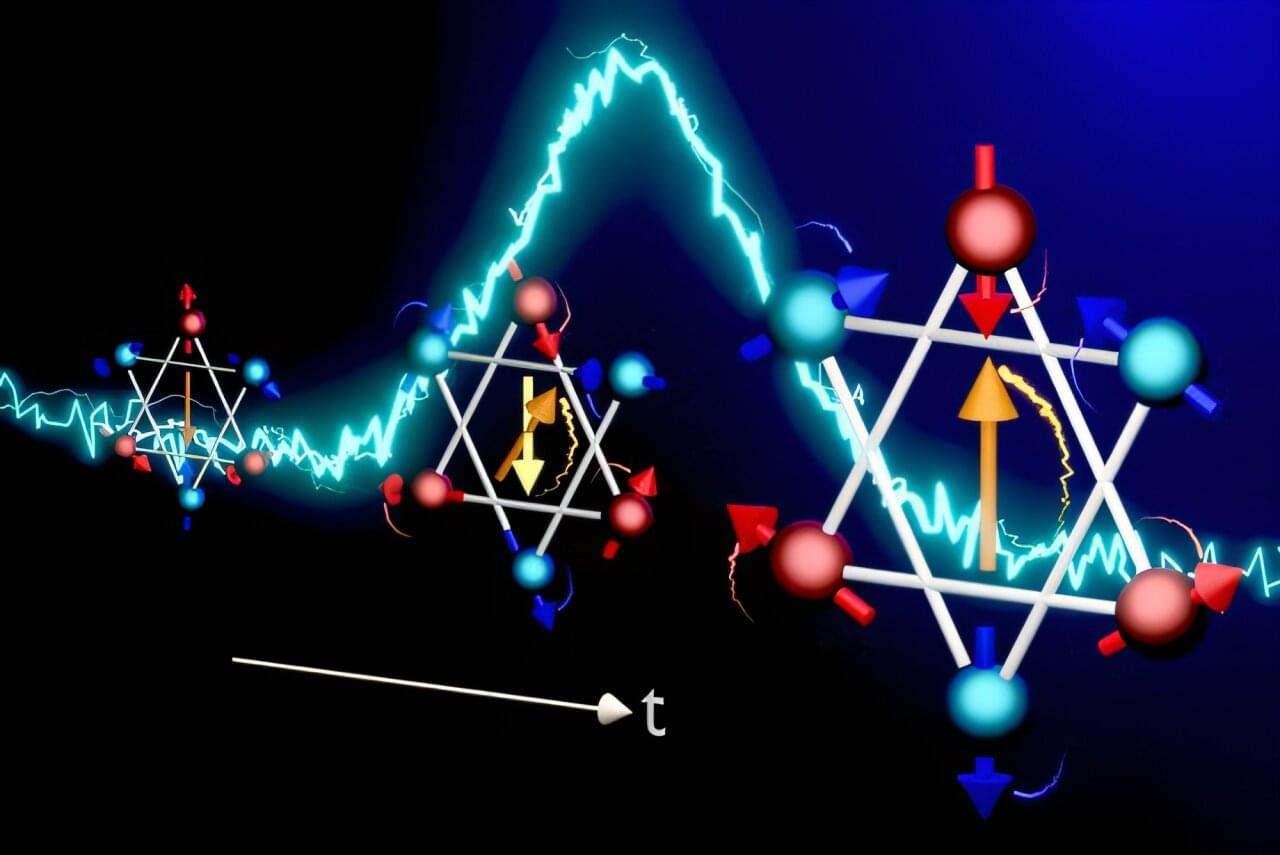
A research team led by Ryo Shimano of the University of Tokyo has successfully visualized two distinct mechanisms through which up and down spins, inherent properties of electrons, switch in an antiferromagnet, a material in which spin alignments cancel each other out. One of the visualized mechanisms provides a working principle for developing ultrafast, non-volatile magnetic memory and logic devices, which could be much faster than today’s technologies.
The findings are published in the journal Nature Materials.
Paper slips with holes, small metal rods, vacuum tubes, and transistors: These are technologies that have been used to encode 0s and 1s, the basis of classical computation. However, the world’s ever-growing computational needs demand yet more powerful tools. Antiferromagnets are a class of materials whose magnetic properties, or lack thereof, could be leveraged to encode 0s and 1s in a novel way.