Circa 2021 Immortality of the male genitalia in humans.
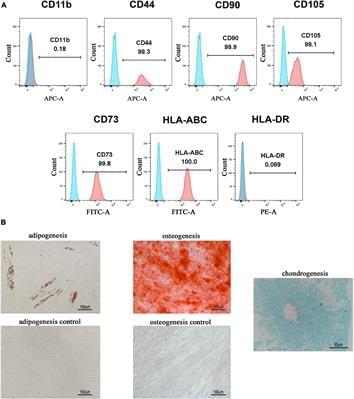
Cavernous nerve injury (CNI) is the main cause of erectile dysfunction (ED) following pelvic surgery. Our previous studies have demonstrated that transplantation of different sources of mesenchymal stem cells (MSCs) was able to alleviate ED induced by CNI in rat models. However, little is known about the therapeutic effects of human gingiva-derived MSCs (hGMSCs) in CNI ED rats. Herein, we injected the hGMSCs around the bilateral major pelvic ganglia (MPG) in a rat model of CNI and evaluated their efficacy. The results showed that treatment of hGMSCs could significantly promote the recovery of erectile function, enhance smooth muscle and endothelial content, restore neuronal nitric oxide synthase (nNOS) expression, and attenuate cell apoptosis in penile tissue. Moreover, penile fibrosis was significantly alleviated after hGMSC administration. In addition, potential mechanism exploration indicated that hGMSCs might exert its functions via skewed macrophage polarity from M1 toward M2 anti-inflammatory phenotype. In conclusion, this study found that transplantation of hGMSCs significantly improved CNI-related ED, which might provide new clues to evaluate their pre-clinical application.
There are many causes of erectile dysfunction (ED), which include psychological factors, neurological disorders (such as multiple sclerosis, temporal lobe epilepsy, and cavernous nerve injury), and vasculogenic disorders (such as atherosclerosis, hypertension, and diabetes mellitus). Neurogenic sexual dysfunction makes up about 10–19% in all causes of erectile dysfunction. Neurotic erectile dysfunction is one of most important complications after radical prostatectomy and rectectomy, owing to intraoperative damage of the pelvic cavernous nerve (CN). It affects not only the physical but also mental health in postoperative patients. Despite the improvement of nerve-sparing techniques, the incidence of neurotic ED still has no substantial improvement. The incidences of ED range from 75 to 80% after pelvic surgery (Schauer et al., 2015).