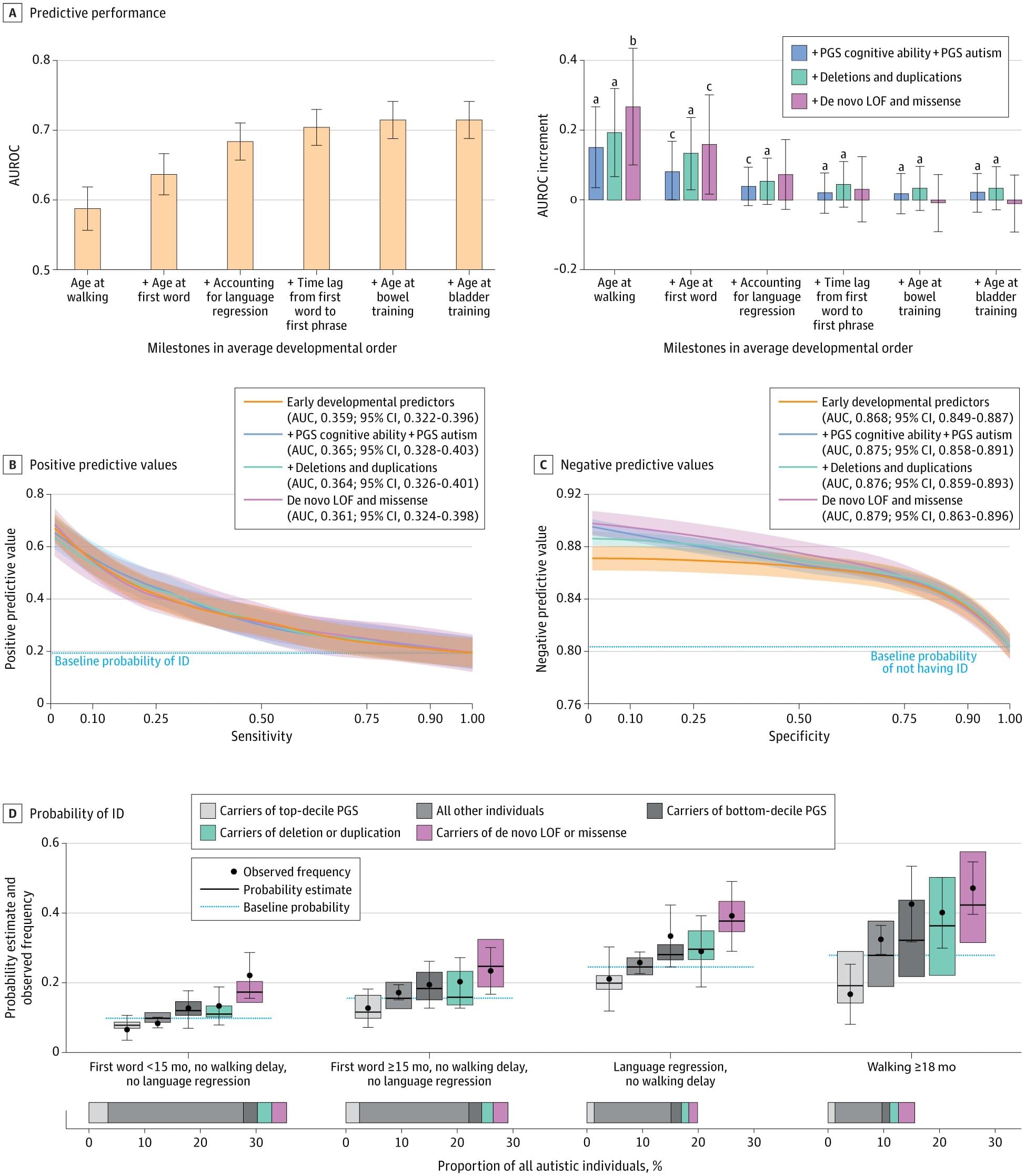
Genome editing has advanced at a rapid pace with promising results for treating genetic conditions—but there is always room for improvement. A new paper by investigators from Mass General Brigham showcases the power of scalable protein engineering combined with machine learning to boost progress in the field of gene and cell therapy.
In their study, the authors developed a machine learning algorithm—known as PAMmla—that can predict the properties of approximately 64 million genome-editing enzymes. The work could help reduce off-target effects and improve editing safety, enhance editing efficiency, and enable researchers to predict customized enzymes for new therapeutic targets. The results are published in Nature.
“Our study is a first step in dramatically expanding our repertoire of effective and safe CRISPR-Cas9 enzymes. In our manuscript, we demonstrate the utility of these PAMmla-predicted enzymes to precisely edit disease-causing sequences in primary human cells and in mice,” said corresponding author Ben Kleinstiver, Ph.D., Kayden-Lambert MGH Research Scholar associate investigator at Massachusetts General Hospital (MGH).