The strong links between changes in astrocyte structure and function in the context of neurodevelopment and disease have been supported by studies examining astrocyte cytoskeletal markers such as glial fibrillary acidic protein (GFAP) in disease models and postmortem human brain tissue, where increases or decreases in its expression in various brain nuclei are often linked with neurocognitive and psychiatric disorders. Hence, changes in GFAP expression are often the first-line test for astrocyte involvement in disease and support a role for astrocyte dysfunction in major depression, schizophrenia, alcohol and substance use disorders, anorexia nervosa, and bipolar disorder (7–19), where changes in astrocyte structure, density, complexity, and/or blood vessel association are linked with disrupted astrocyte function. Although reactive astrogliosis remains the single most studied astrocytic response involving morphological adaptations and changes in GFAP expression (20, 21), in recent years, astrocyte morphological plasticity has been shown to be more nuanced. GFAP expression is dynamic across the circadian cycle (22–24) and increases with physical exercise and environmental enrichment (25, 26). Moreover, in aging, astrocytes increase or decrease their GFAP expression in different brain regions (27, 28), suggesting heterogeneity in astrocyte form and function.
We previously found a notable relationship between astrocyte structure and vulnerability to substance use disorders, with astrocytes in the nucleus accumbens (NAc) altering their association with different neural subcircuits to drive or suppress drug-seeking behavior depending on heroin availability (29–31). The NAc is critical for regulating behavioral outputs in response to rewards, including substances of abuse and natural reinforcers, such as food or sucrose. The NAc is composed of core and shell subregions that are themselves heterogeneous structures with regard to synaptic input and output connectivity and function (32–36). Heterogeneity has been observed in astrocyte morphology within the NAc core (3, 30, 37), but studies have not yet examined how astrocyte structure and function differ across NAc subregions at baseline or in response to operant conditioning with natural or pathological reinforcers.
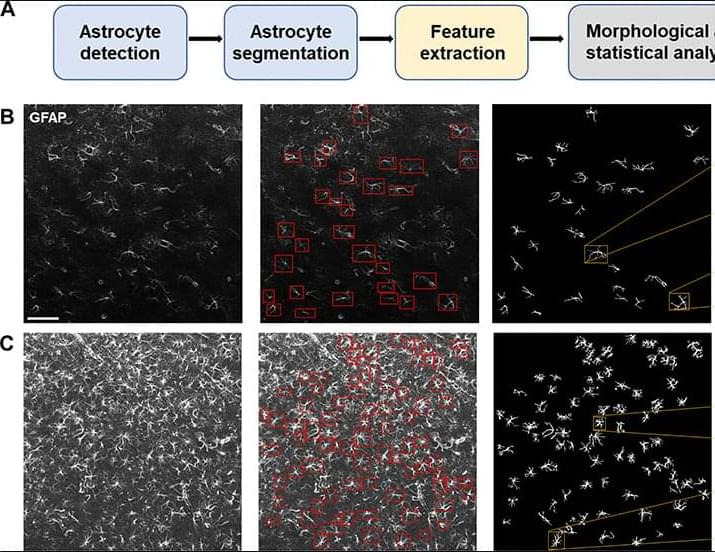
To address this gap, we developed an automated pipeline for single-cell morphological analysis of astrocytes that integrates state-of-the-art deep learning models for astrocyte detection and segmentation, together with highly sensitive geometrical tools for precise quantitation of single-cell morphological characteristics. We introduce the rigorous notion of morphological distance (MD) to measure alterations in astrocyte morphology and compare astrocyte subpopulations according to their structural characteristics. By applying this pipeline in combination with supervised machine learning, we found that single-astrocyte morphological characteristics were predictive not only of anatomical location within the NAc at baseline but also of the availability of heroin or sucrose at the moment of image capture. This geometrically sensitive approach yields substantially more detailed information about astrocyte structure than previously applied manual or semiautomated approaches and serves as a rigorous quantitative assay for identifying brain nuclei where astrocytes undergo plasticity in the context of disease. We found that astrocyte structural plasticity across the NAc was disrupted in animals that had been exposed to heroin but not sucrose, consistent with a largely protective role for NAc astrocytes in maintaining synaptic homeostasis and behavioral flexibility. We also found that astrocyte structural plasticity in the dorsomedial portion of the NAc shell was uniquely engaged during the initiation of opioid but not sucrose seeking, suggesting the involvement of this structure in drug relapse.