Circa 2016
Reports that scientists have created “the first fully living dinosaur embryo in millions of years” using DNA from chicken skin are fake news.


No one should think we are over COVID.
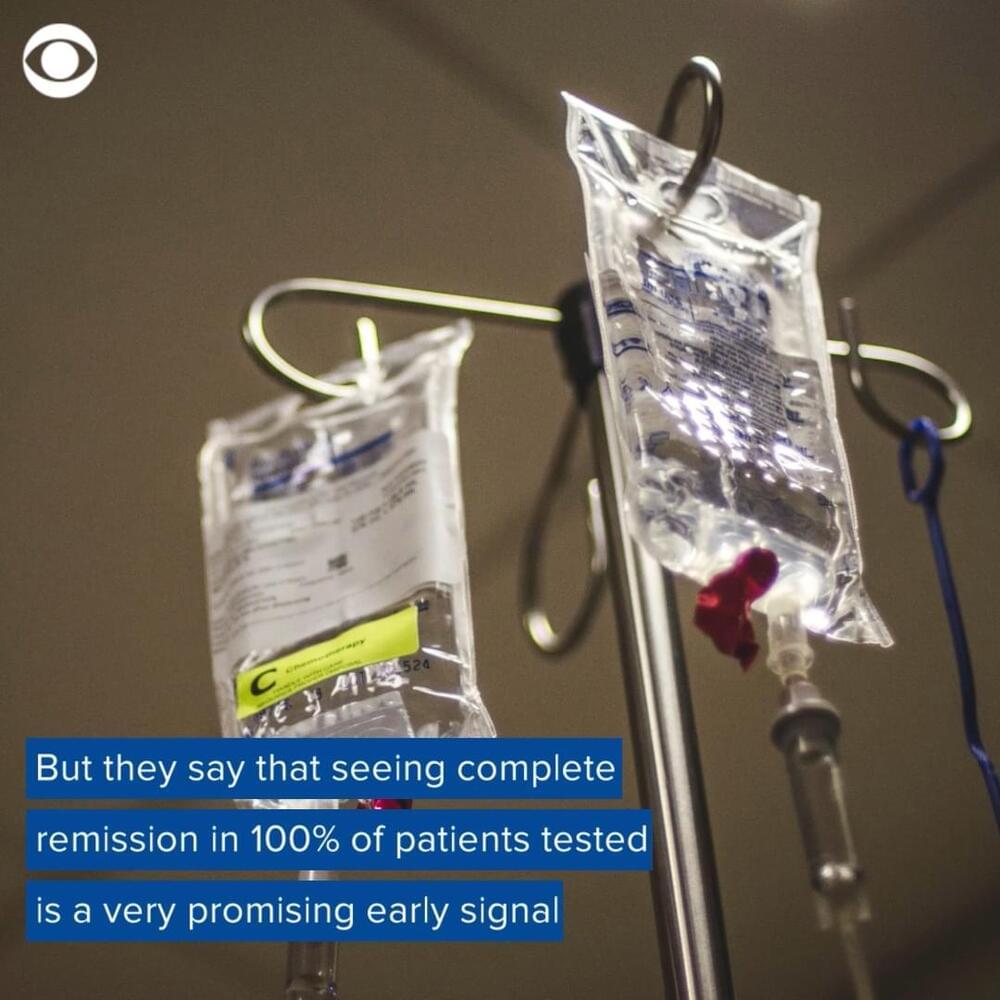
WASHINGTON — Federal health officials warned on Wednesday that a third of Americans live in areas where the threat of Covid-19 is now so high that they should consider wearing a mask in indoor public settings. They cited new data showing a substantial jump in both the spread of the coronavirus and hospitalizations over the past week.
Dr. Rochelle P. Walensky, the director of the Centers for Disease Control and Prevention, said that the seven-day average of hospital admissions from Covid rose 19 percent over the previous week. About 3,000 people a day were being admitted with Covid, she said, although death rates, a lagging indicator, remained low.
More than 32 percent of Americans now live in counties with medium to high levels of virus transmission, compared with about 24 percent the previous week. Dr. Walensky said that local leaders and individuals in those regions should adopt — or at least consider — prevention strategies, such as masking in indoor public settings and more frequent testing.
Join us on Patreon!
https://www.patreon.com/MichaelLustgartenPhD
Cronometer Discount Link:
https://shareasale.com/r.cfm?b=1390137&u=3266601&m=61121&urllink=&afftrack=
Papers referenced in the video:
Glycine supplementation extends lifespan of male and female mice.
https://pubmed.ncbi.nlm.nih.gov/30916479/
Ergothioneine exhibits longevity-extension effect in Drosophila melanogaster via regulation of cholinergic neurotransmission, tyrosine metabolism, and fatty acid oxidation.
https://pubmed.ncbi.nlm.nih.gov/34877949/
17-a-estradiol late in life extends lifespan in aging UM-HET3 male mice; nicotinamide riboside and three other drugs do not affect lifespan in either sex.
https://pubmed.ncbi.nlm.nih.gov/33788371/
Metagenomic and metabolomic remodeling in nonagenarians and centenarians and its association with genetic and socioeconomic factors.

By making remarkable breakthroughs in a number of fields, unlocking new approaches to science, and accelerating the pace of science and innovation.
In 2020, Google’s AI team DeepMind announced that its algorithm, AlphaFold, had solved the protein-folding problem. At first, this stunning breakthrough was met with excitement from most, with scientists always ready to test a new tool, and amusement by some. After all, wasn’t this the same company whose algorithm AlphaGo had defeated the world champion in the Chinese strategy game Go, just a few years before? Mastering a game more complex than chess, difficult as that is, felt trivial compared to the protein-folding problem. But AlphaFold proved its scientific mettle by sweeping an annual competition in which teams of biologists guess the structure of proteins based only on their genetic code. The algorithm far outpaced its human rivals, posting scores that predicted the final shape within an angstrom, the width of a single atom. Soon after, AlphaFold passed its first real-world test by correctly predicting the shape of the SARS-CoV-2 ‘spike’ protein, the virus’ conspicuous membrane receptor that is targeted by vaccines.
The success of AlphaFold soon became impossible to ignore, and scientists began trying out the algorithm in their labs. By 2021 Science magazine crowned an open-source version of AlphaFold the “Method of the Year.” Biochemist and Editor-in-Chief H. Holden Thorp of the journal Science wrote in an editorial, “The breakthrough in protein-folding is one of the greatest ever in terms of both the scientific achievement and the enabling of future research.” Today, AlphaFold’s predictions are so accurate that the protein-folding problem is considered solved after more than 70 years of searching. And while the protein-folding problem may be the highest profile achievement of AI in science to date, artificial intelligence is quietly making discoveries in a number of scientific fields.
By turbocharging the discovery process and providing scientists with new investigative tools, AI is also transforming how science is done. The technology upgrades research mainstays like microscopes and genome sequencers 0, adding new technical capacities to the instruments and making them more powerful. AI-powered drug design and gravity wave detectors offer scientists new tools to probe and control the natural world. Off the lab bench, AI can also deploy advanced simulation capabilities and reasoning systems to develop real-world models and test hypotheses using them. With manifold impacts stretching the length of the scientific method, AI is ushering in a scientific revolution through groundbreaking discoveries, novel techniques and augmented tools, and automated methods that advance the speed and accuracy of the scientific process.

A new molecule synthesized by a University of Texas at Dallas researcher kills a broad spectrum of hard-to-treat cancers, including triple-negative breast cancer, by exploiting a weakness in cells not previously targeted by other drugs.
A study describing the research — which was carried out in isolated cells, in human cancer tissue and in human cancers grown in mice — was published online June 2 in the journal Nature Cancer.
Dr. Jung-Mo Ahn, a co-corresponding author of the study and a UT Dallas associate professor of chemistry and biochemistry in the School of Natural Sciences and Mathematics, has been passionate about his work designing small molecules that target protein-protein interactions in cells for over a decade. Using an approach called structure-based rational drug design, he previously developed potential therapeutic candidate compounds for treatment-resistant breast cancer and for prostate cancer.
Liz mentions combinatorial gene therapy for aging near the end which is something you hear the likes of George Church mention they are working on.
Liz Parrish is the founder of @BioViva Science, a company dedicated to curing biological aging, a disease that is at the root cause of all other chronic diseases from heart disease to Alzheimer’s. Watch this video to understand how much more control we have over our lifespan and health!
💻Connect with BioViva here:
Website: https://bioviva-science.com/
YouTube channel: https://www.youtube.com/channel/UCaBq8hEExcUN6mtKMEuBvMQ
Instagram: https://www.instagram.com/biovivasciences/
Twitter: https://twitter.com/BioVivaScience.
Facebook: https://www.facebook.com/BiovivaSciences/
💻Connect with Liz Parrish here:
Instagram: https://www.instagram.com/lizlparrish/
Twitter: https://twitter.com/parrishliz?lang=en.
☕EAT MEAT. LIFT. REPEAT. mug merch: https://bit.ly/3GuFUXx.

The reluctance of many in the medical field to classify aging as a disease is causing significant roadblocks for those trying to find a solution.
Many people will swear to the life extending properties of coffee, be it saving them from keeling over from exhausting in the early hours of the morning or saving an annoying co-worker from the unbridled rage of someone who hasn’t yet acquired their caffeine fix. Yes, coffee is without a doubt one of the most powerful (and mostly metaphorical) lifesavers of the modern world. However, recent studies into the effects of drinking coffee on human lifespan have found that it might very well have a significant impact on health and longevity. A study of 170,000 people from the UK found that those who drank between two and four cups of coffee a day were 30% less likely to die from all causes compared to those who did not drink coffee at all.