The DNA-altering technology has revolutionized life sciences research and is making strides in potential one-and-done treatments for sickle cell disease, genetic liver conditions, and more.


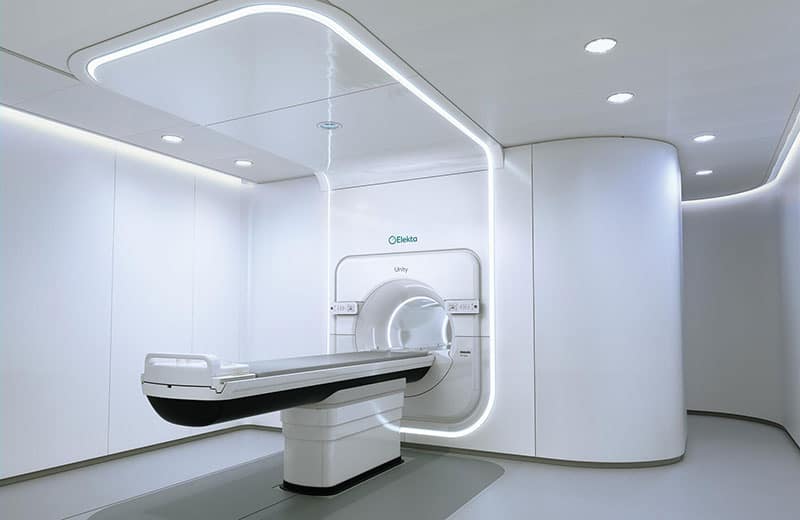
Join the audience for a live webinar at 3 p.m. BST on 5 July 2022 exploring the most recent real-time adaptive radiotherapy developments and the new cardiac radioablation treatment.
Stereotactic radioablation is a novel, non-invasive treatment option for cardiac arrhythmias. The heart is dose sensitive and its motion contributes significantly to dose delivery uncertainties.
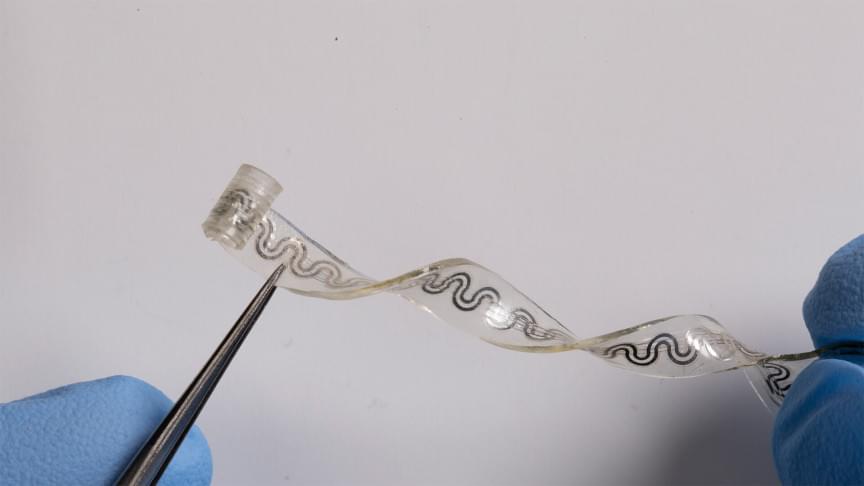
A Northwestern University-led team of researchers has developed a small, soft, flexible implant that relieves pain on demand and without the use of drugs. The first-of-its-kind device could provide a much-needed alternative to opioids and other highly addictive medications.
In the future, a woman with a spinal cord injury could make a full recovery; a baby with a weak heart could pump his own blood. How close are we today to the bold promise of bionics—and could this technology be used to improve normal human functions, as well as to repair us? Join Bill Blakemore, John Donoghue, Jennifer French, Joseph J. Fins, and P. Hunter Peckham at “Better, Stronger, Faster,” part of the Big Ideas Series, as they explore the unfolding future of embedded technology.
This program is part of the Big Ideas Series, made possible with support from the John Templeton Foundation.
Visit our Website: http://www.worldsciencefestival.com/
Like us on Facebook: https://www.facebook.com/worldscience… us on twitter: https://twitter.com/WorldSciFest Original Program date: May 31, 2014 Host: Bill Blakemore Participants: John Donoghue, Jennifer French, Joseph J. Fins, P. Hunter Peckham Re-engineering the anatomy of the “Vitruvian Man” 00:00 Bill Blakemore’s Introduction. 2:06 Participant introductions. 4:27 What is FES? (Functional Electrical Stimulation) 6:06 A demonstration with FES and without. 10:06 How did you test FES systems? 14:16 Jen French the first bionic pioneer. 16:40 What was the journey like from injury to today? 18:35 A live demonstration of FES. 20:40 What is BrainGate? 27:55 What is the potential for this technology? 37:00 When will this technology be publicly available? 40:50 A cell phone app to drink water or stand up? 44:55 Jen French would be the first to try new technology. 50:39 What is the history of altering the human brain? 1:00:57 The move from chemical to electrical medical care. 1:05:40 The challenge of what is going to drive the delivery of care to groups in need. 1:11:36 Can these devices be implanted without surgery? 1:18:13 What field needs the most funding for this to become available to everyone? 1:19:40 What are the numbers of people who can use this technology? 1:23:44 Why can’t we use stem cells to reconnect human spinal tissue? 1:25:37 What is the collaboration level between institutions? 1:29:16 How far away are we from using brain waves to control objects and communicate with each other? 1:30:20
Follow us on twitter: https://twitter.com/WorldSciFest.
Original Program date: May 31, 2014
Host: Bill Blakemore.
Participants: John Donoghue, Jennifer French, Joseph J. Fins, P. Hunter Peckham.
Re-engineering the anatomy of the “Vitruvian Man” 00:00.
Bill Blakemore’s Introduction. 2:06
For decades, biologists have read and edited DNA, the code of life. Revolutionary developments are giving scientists the power to write it. Instead of tinkering with existing life forms, synthetic biologists may be on the verge of writing the DNA of a living organism from scratch. In the next decade, according to some, we may even see the first synthetic human genome. Join a distinguished group of synthetic biologists, geneticists and bioengineers who are edging closer to breathing life into matter.
This program is part of the Big Ideas Series, made possible with support from the John Templeton Foundation.
Original Program Date: June 4, 2016
MODERATOR: Robert Krulwich.
PARTICIPANTS: George Church, Drew Endy, Tom Knight, Pamela Silver.
Visit our Website: http://www.worldsciencefestival.com/
Like us on Facebook: https://www.facebook.com/worldsciencefestival.
Follow us on twitter: https://twitter.com/WorldSciFest.
Synthetic Biology and the Future of Creation 00:00.
Participant Intros 3:25
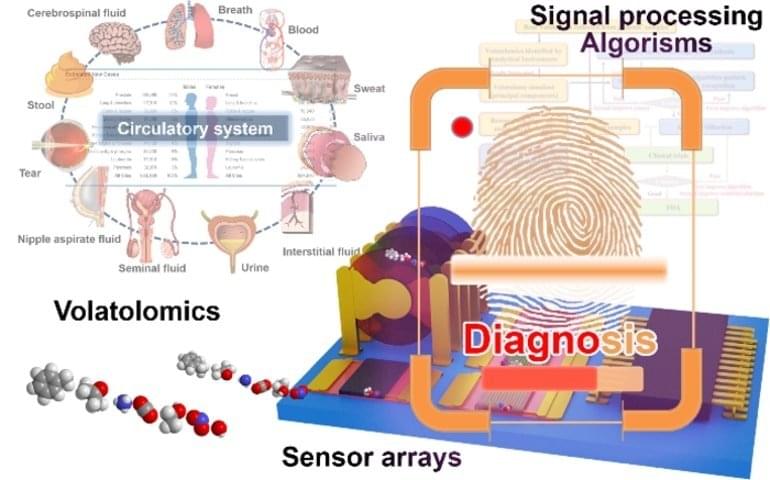
Summary: A new robotic system can identify volatile organic compounds associated with diseases by analyzing bodily emissions.
Source: Tsinghua University Press.
Scientists are working on diagnostic techniques that could sniff out chemical compounds from breath, sweat, tears and other bodily emissions and that act as fingerprints of thousands of diseases.
Metaverse though considered, a world under construction, has already created exciting promises. An individual can replicate his identity and even enhance them. How is it possible for a virtual world to create the exact replica of a person in zeroes and ones? There is not just one technology aiding in creating the fascinating world of Metaverse and IoT is one amongst them.
IoT connects digital devices via sensors and gadgets. It connects voice-activated speakers, medical gadgets, thermostats, and weather sensors, to data sources. Metaverse’s IoT applications collect and distribute data from the physical world to create an accurate representation of an object. A person’s replica in a Metaverse might have a unique biophysical response for example when the real person relocates to a place with different weather.
3D environments become easy and seamless to adapt in Metaverse as it connects a range of real-life devices through IoT. Making simulations within the Metaverse, particularly with digital twins becomes a lot easier making the physical and digital worlds indistinguishable all while providing a tailored interface environment for IoT. For example, with the gaming interface, elevated heart and breathing rates can trigger the individual’s avatar to make it more susceptible to replicating the person in real.
A look at the concept of Self-Replicating Machines, Universal Assemblers, von Neumann Probes, Grey Goo, and Berserkers. While we will discuss the basic concept and some on-Earth applications like Medical Nanotechnology our focus will be on space exploration and colonization aspects.
Join the Facebook Group:
https://www.facebook.com/groups/1583992725237264/
Visit our Website:
www.IsaacArthur.net.
Support the Channel on Patreon:
https://www.patreon.com/IsaacArthur.
Listen or Download the audio of this episode from Soundcloud:
https://soundcloud.com/isaac-arthur-148927746/https://soundc…machines-1
Cover Art by Jakub Grygier: