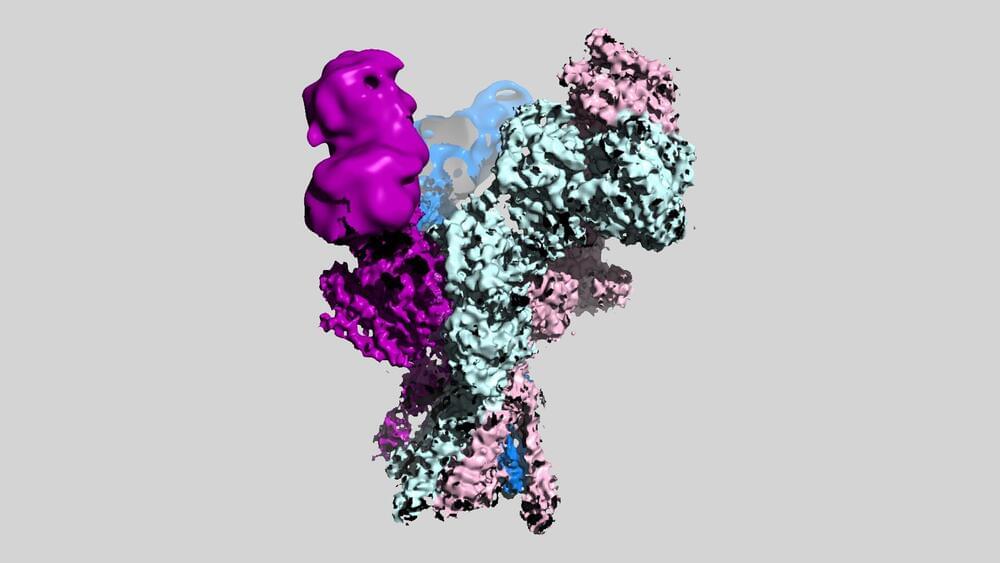
New images from scientists at Cold Spring Harbor Laboratory (CSHL) reveal for the first time the three-dimensional structures of a set of molecules critical for healthy brain function.
The molecules are members of a family of proteins in the brain known as NMDA receptors, which mediate the passage of essential signals between neurons. The detailed pictures generated by the CSHL team will serve as a valuable blueprint for drug developers working on new treatments for schizophrenia, depression, and other neuropsychiatric conditions.
“This NMDA receptor is such an important drug target,” says Tsung-Han Chou, a postdoctoral researcher in CSHL Professor Hiro Furukawa’s lab. That’s because dysfunctional NMDA receptors are thought to contribute to a wide range of conditions, including not just depression and schizophrenia, but also Alzheimer’s disease, stroke, and seizures. “We hope our images, which visualize the receptor for the first time, will facilitate drug development across the field based on our structural information,” Chou says.