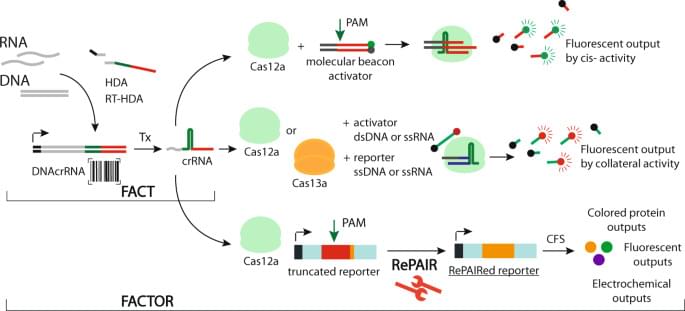
Genetic Engineering extends far beyond the controversial news headlines that obsess over ‘designer babies’. In the science community, gene-editing tools like CRISPR and PRIME editing will do nothing less than save the planet.
The Rise Of Genetic Engineering (2022)
Writers: Kyle McCabe, Christopher Webb Young.
Stars: Rodolphe Barrangou, George Church, Mary Beth Dallas.
Genre: Documentary.
Country: United States.
Language: English.
Release Date: August 24, 2022 (United States)
Synopsis:
Genetic Engineering extends far beyond the controversial news headlines that obsess over ‘designer babies’. In the science community, gene-editing tools like CRISPR and PRIME editing will do nothing less than save the planet.
Methods like this allow scientists to alter and ‘re-program’ the genetics of living organisms.
This episode shows scientists at large using gene-editing technologies to revolutionize the food supply chain, bolstering food crops to prevent famines, and even speed up reforestation efforts that will reverse global warming. Genetic Engineering in farm animals is helping scientists to ‘select’ desirable traits, like physical features and gender. Incredibly, one scientist is using gene-editing technologies to resurrect the DNA of extinct species, like the Wooly Mammoth!
Despite some public concern, gene-editing is definitely a cause for hope in the fight against genetic disorders in humans. It’s already reversing a type of congenital blindness in children. And with the hyper-precision afforded by PRIME editing being prepared for clinical trials, a much more hopeful world will be revealed for families in the future.