Year 2020 face_with_colon_three
Ah, Memories! They can be some of our best assets or our most painful tormentors. Good memories give us a sensation of warmth and hope for better times, but bad memories can cause serious trauma.
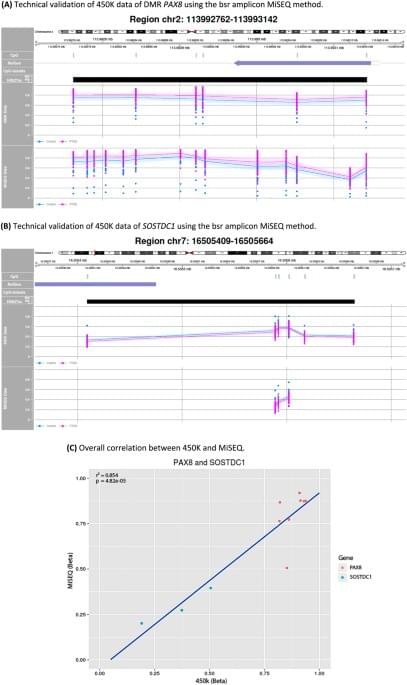
In this study we aimed to detect epigenetic and genetic loci associated with PTSD in a homogeneous cohort of traumatized police officers. Both a genome-wide and hypothesis-driven replication approach did not result in DMPs between PTSD patients and trauma-exposed controls. GSE analysis on the top 100 DMPs showed, however, a plausible association of the dopaminergic neurogenesis pathway with PTSD. Furthermore, we observed one DMR located at the PAX8 gene suggesting consistent hypermethylation in PTSD patients. Genetic analyses yielded three CpG-SNPs significantly associated with PTSD. Of these, one CpG-SNP, located at the CACNA1C locus, was also significantly associated with PTSD in an independent replication sample of trauma-exposed children. Notably, this result shows that the Illumina 450K array is not restricted to epigenetic surveys but can provide informative genetic data as well.
Although our sample was small, it was highly homogenous as all participants were former or current police officers, and cases and controls were matched for sex, age, education, and years of police service. All participants reported multiple prior traumatic events, without significant group differences in reported types of traumatic experiences. PTSD patients fulfilled current diagnostic criteria for PTSD, while our trauma-exposed controls had minimal PTSD symptoms and did not report lifetime PTSD or other trauma-related psychiatric disorders. Thus our controls were apparently resilient to adverse mental health outcome of trauma. This study design, including extreme phenotypes following similar trauma load, was considered to favor detection of PTSD-associated loci, as also suggested by others [22]. Nevertheless, our genome-wide survey clearly remains limited in statistical power.
Men with high-grade prostate cancer and low prostate-specific antigen (PSA) levels have a poor prognosis. The question remains as to whether the chemotherapy drug docetaxel, which increases survival in metastatic prostate cancer, can improve the cure rate in these patients.
In a new study by investigators from Brigham and Women’s Hospital, a meta-analysis of five prospective randomized clinical trials (RCTs) found that adding docetaxel to standard-of-care (SOC) treatment was associated with a 70% reduction in death from prostate cancer-specific mortality (PCSM). The study was published today in JAMA Network Open.
Investigators performed a meta-analysis of the RCTs evaluating SOC treatment with radiotherapy and androgen deprivation therapy or with radical prostatectomy versus SOC plus docetaxel. The final study cohort of 2,184 patients included 145 eligible patients (6.6%) across four eligible RCTs.
Triple-negative breast cancer (TNBC) is an aggressive tumor with a very poor prognosis and limited therapeutic targets. Now, researchers at Baylor College of Medicine and collaborating institutions have discovered in diverse TNBC animal models that targeting the protein elF4A with the small molecule drug Zotatifin suppressed tumor cell proliferation and remodeled the tumor immune microenvironment. The findings may lead to clinical trials to assess the potential patient benefits of this novel approach.
The team published their findings in The Journal of Clinical Investigation in an article titled, “Targeting EIF4A triggers an interferon response to synergize with chemotherapy and suppress triple-negative breast cancer.”
“Protein synthesis is frequently dysregulated in cancer and selective inhibition of mRNA translation represents an attractive cancer therapy,” wrote the researchers. “Here, we show that therapeutically targeting the RNA helicase eIF4A by Zotatifin, the first-in-class eIF4A inhibitor, exerts pleiotropic effects on both tumor cells and the tumor immune microenvironment in a diverse cohort of syngeneic triple-negative breast cancer (TNBC) mouse models.”

Emmett Short discusses these comments on this episode of Lifespan News.
But first, the mad scientist David Sinclair, this time with Peter Diamandis at Abundance 360, giving more details into human trials for the genetic engineering side of the technology versus the chemical and pill side of the technology. Which would you want more? We’ll also hear David’s thoughts on how AI will affect the advancement of this tech. Spoiler: A lot. I’m going to play the best parts and add my commentary along the way.
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links:
Telomere and Epigenetic Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1
Use Code: CONQUERAGING
NAD+ Quantification: https://www.jinfiniti.com/intracellular-nad-test/
Use Code: ConquerAging At Checkout.
Oral Microbiome: https://www.bristlehealth.com/?ref=michaellustgarten.
Enter Code: ConquerAging.
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
At-Home Blood Testing (SiPhox Health): https://getquantify.io/mlustgarten.

Music for pain relief and anxiety. I think somewhere else I read it helps heal brain injuries.
Further, subject-preferred music appears to induce a superior effect in relieving pain. This can be approached by allowing participants to select the most pleasant music from a prespecified list of songs or listen to their favorite music during the study. Nevertheless, the richness of emotions, meanings, and associations involved when listening to favorite music is poorly understood, especially regarding pain relief.
About the study
In the present study, researchers evaluated which subjective aspects of listening to relaxing and favorite music were crucial for hypoalgesia. Sixty-three healthy individuals, aged 21.3, on average, participated in this study. A thermal contact probe was used to induce painful thermal stimuli on the inner forearm surface.
Cancer patients receiving anthracycline chemotherapy have more than twice the risk of developing heart failure compared to their peers without cancer.1The ground-breaking RESILIENCE project aims to prevent heart failure in patients who need anthracyclines to treat their cancer.
International experts and patients from the project gathered yesterday at the European Heart House, home of the European Society of Cardiology (ESC), as a unique think tank to improve the healthcare and quality of life for patients with cancer.
It is estimated that four million Europeans are diagnosed with cancer every year.2 Anthracycline chemotherapy has a prominent role in treating many forms of cancer-for example, up to 70% of patients with lymphoma receive an anthracycline regimen. Currently, there is no therapy to prevent anthracycline cardiotoxicity.
The Wistar Institute’s David B. Weiner and collaborators have engineered novel monoclonal antibodies that engage natural killer (NK) cells through a unique surface receptor that activates the immune system to fight against cancer.
In their publication titled, “Siglec-7 glyco-immune binding MAbs or NK cell engager biologics induce potent anti-tumor immunity against ovarian cancers,” published in Science Advances, the team demonstrates the preclinical feasibility of utilizing these new cancer immunotherapeutic approaches against diverse ovarian cancer types, including treatment-resistant and refractory ovarian cancers—alone or in combination with checkpoint inhibitor treatment.
The research started as a collaboration between Wistar’s Drs. Weiner and Mohamed Abdel-Mohsen, who were exploring the development of new glyco-signaling biologic tools that may be important in the fight against cancer.
Lung cancer is the third most common cancer in the U.S. and there have been more than 235,000 new cases of lung cancer in 2021. While this figure is significant, the rate of new lung and bronchus cancer cases is decreasing, in part because more people have stopped smoking. This trend, along with innovations in early detection and treatment, is also reducing the number of lung cancer deaths.
Dr. Robert Taylor Ripley, associate professor of surgery in the Division of General Thoracic Surgery, is an expert in mesothelioma and thoracic surgical oncology. In the following Q&A, he discusses common causes of lung cancer, risks and the latest treatments.
Q: What are the most common causes of lung cancer? A: Smoking cigarettes is the most common cause, but others include secondhand smoke and environmental inhalants. We see a fair number of patients with lung cancer who have never smoked. Exposure to diesel exhaust or other chemicals may also cause lung cancer in some non-smokers.