Smartphone apps can differentiate between tuberculosis and other respiratory conditions. It’s part of an AI-driven trend: using sound to diagnose illnesses.



Researchers from Harvard SEAS and Boston University reveal its transformative effects, offering newfound mobility and independence for individuals with this debilitating condition.
The wearable tech successfully eliminates a common symptom called ‘gait freezing’ to restore smooth strides for Parkinson’s disease sufferers.

😀 They say we could even regenerate human limbs this way aswell as repair human blood vessels.
Cell tubes, made entirely from a patient’s own cells, are just as elastic as blood vessels but much stronger. Skin cells cultured into lumps are skewered on needles on a base, similar to a Kenzan, a tool used in Japanese flower arrangements, and formed into a tube. The technique, called the Kenzan Method, was made possible by a 3D bioprinter. A clinical trial is underway in Japan to transplant these tubes into humans in place of blood vessels. Studies are being done to apply them to nerves and organs.

How deeply someone can be hypnotized — known as hypnotizability — appears to be a stable trait that changes little throughout adulthood, much like personality and IQ. But now, for the first time, Stanford Medicine researchers have demonstrated a way to temporarily heighten hypnotizablity — potentially allowing more people to access the benefits of hypnosis-based therapy.
In the new study, published Jan. 4 in Nature Mental Health, the researchers found that less than two minutes of electrical stimulation targeting a precise area of the brain could boost participants’ hypnotizability for about one hour.
“We know hypnosis is an effective treatment for many different symptoms and disorders, in particular pain,” said Afik Faerman, PhD, a postdoctoral scholar in psychiatry and lead author of the study. “But we also know that not everyone benefits equally from hypnosis.”
From surveillance to defense to AI/ML virtualization, and it’s more compact and energy efficient. Oh and let’s not forget the medical imaging applications. I just wonder how long until it’s put into effect.
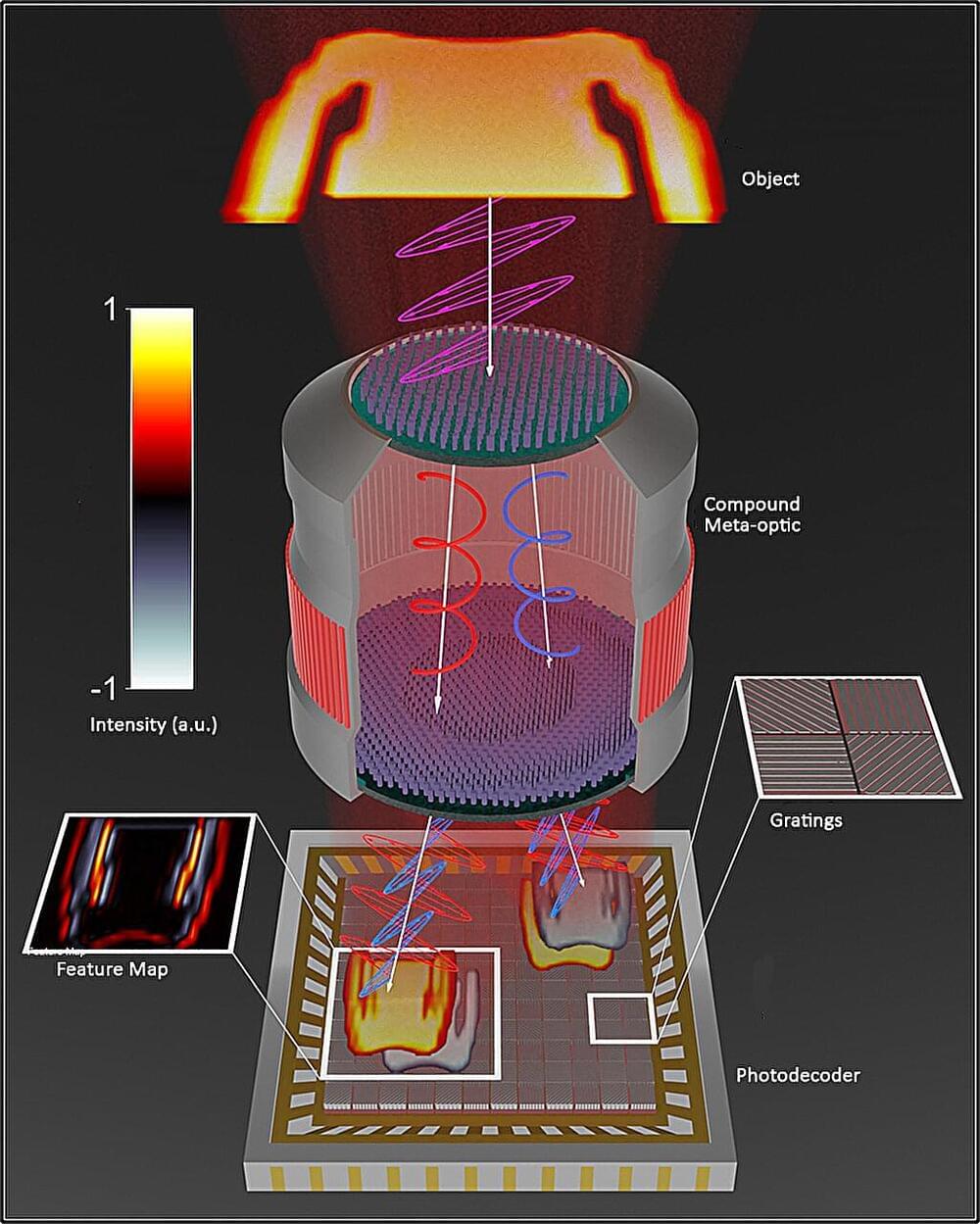
A front-end lens, or meta-imager, created at Vanderbilt University can potentially replace traditional imaging optics in machine-vision applications, producing images at higher speed and using less power.
The nanostructuring of lens material into a meta-imager filter reduces the typically thick optical lens and enables front-end processing that encodes information more efficiently. The imagers are designed to work in concert with a digital backend to offload computationally expensive operations into high-speed and low-power optics. The images that are produced have potentially wide applications in security systems, medical applications, and government and defense industries.
Mechanical engineering professor Jason Valentine, deputy director of the Vanderbilt Institute of Nanoscale Science and Engineering, and colleagues’ proof-of-concept meta-imager is described in a paper published in Nature Nanotechnology.
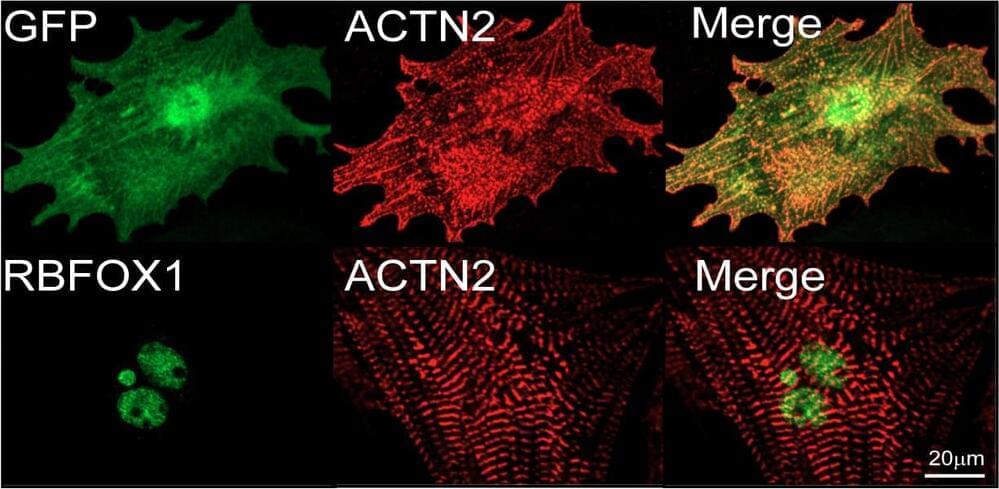
Researchers identify RBFox1 as a key intrinsic regulator of heart muscle cell maturation, overcoming a major limitation in cardiac regenerative therapy and disease modelling and demonstrating for the first time that RNA splicing control can significantly impact this process.
Scientists led by Duke-NUS Medical School in Singapore and the University of California, Los Angeles, (UCLA) in the United States have discovered a new control mechanism that can drive the maturation of human stem cell-derived heart muscle cells, providing fresh insight into the maturation process of heart muscle cells from fetal to adult form.
After birth, heart muscle cells undergo extensive changes to become fully mature adult cells, altering their form, function and physiology.
📸 Look at this post on Facebook https://www.facebook.com/100064499509946/posts/774174594742517/?mibextid=CDWPTG
Partial-heart transplant from a living donor allows an infant’s heart valves to grow as he does.
AI finds a new class of antibiotics.

SYDNEY — With fangs that could pierce a human fingernail, the largest male specimen of the world’s most venomous arachnid has found a new home at the Australian Reptile Park where it will help save lives after a member of the public discovered it by chance.
The deadly Sydney funnel-web spider dubbed “Hercules” was found on the Central Coast, about 50 miles north of Sydney, and was initially given to a local hospital, the Australian Reptile Park said in a statement Thursday.
Spider experts from the nearby park retrieved it and soon realized it was the largest male specimen ever received from the public in Australia.
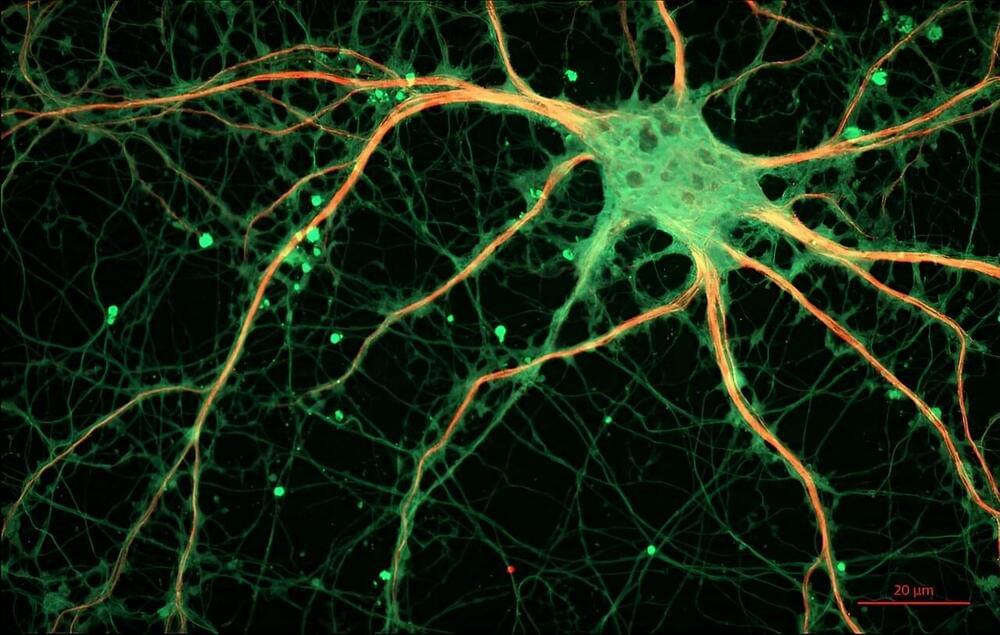
Over the past decades, scientists have made substantial progress unveiling the underlying mechanisms behind many psychiatric disorders. Every year, new genetic mutations or protein dysregulations are identified as potential culprits for the symptoms and sometimes even the root causes of complex neurological diseases, including autism spectrum disorder (ASD), schizophrenia, and Alzheimer’s.
Despite these efforts, the precise roles of several proteins involved in brain function remain obscure. Such is the case for indoleamine 2,3-dioxygenase 2 (IDO2), an enzyme expressed in the brain and metabolized by the tryptophan–kynurenine pathway (TKP).
Changes in the metabolites of this pathway have already been linked to many psychiatric disorders, and genetically modified mice have been invaluable tools in such studies. However, the detailed functions of IDO2 in the brain are not known.