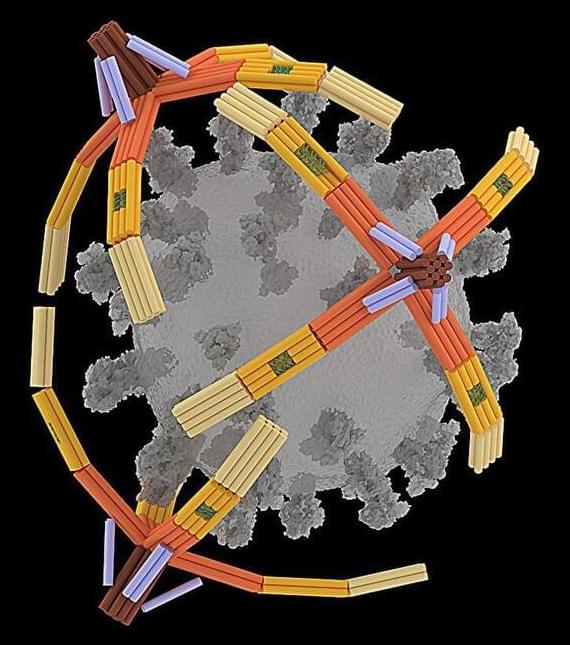
In a new work, a team from the University of Pennsylvania tracked the impact of alcohol consumption from the age of 20 on brain health and came to disappointing conclusions.
UNIVERSITY PARK, Pa. — Binge drinking in early adults can lead to long-lasting and potentially permanent dysregulation in the brain, according to a new study in mice, led by researchers at Penn State. They found that neurons, cells that transmit information in the brain via electrical and chemical signals, showed changes following binge drinking were similar in many ways to those seen with cognitive decline.
These findings, published in the journal Neurobiology of Aging, reveal that binge drinking early in life may have lasting impacts that are predictive of future health issues, like Alzheimer’s disease and related dementias, the researchers said. The work could inform the development of therapeutics to help combat these changes — particularly in aging populations who may have given up alcohol decades earlier, according to Nikki Crowley, director of the Penn State Neuroscience Institute at University Park, Huck Early Career Chair in Neurobiology and Neural Engineering, assistant professor of biology in the Eberly College of Science, and the leader of the research team.
“We know from previous studies that there are immediate effects of binge drinking on the brain, but we didn’t have any sense of if these changes were long-lasting, or reversible over time,” said Crowley, who is also an assistant professor of biomedical engineering and of pharmacology. “We were interested in understanding if binge drinking during early adulthood may have lasting consequences that are not revealed until later in life — even if drinking had stopped for a very long period of time. This allows us to consider the effects of alcohol on an individual’s holistic health, in terms of their entire life history.”