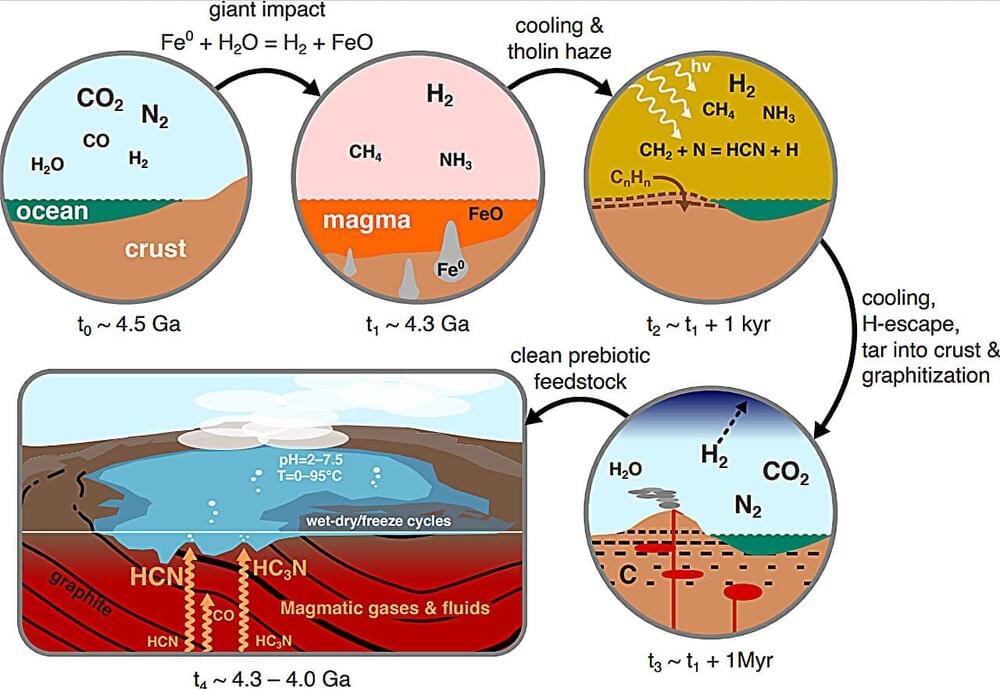
Scientists in Cambridge University suggest molecules, vital to the development of life, could have formed from a process known as graphitization. Once verified in the laboratory, it could allow us to try and recreate plausible conditions for life’s emergence.
How did the chemicals required for life get there? It has long been debated how the seemingly fortuitous conditions for life arose in nature, with many hypotheses reaching dead ends. However, researchers at the University of Cambridge have now modeled how these conditions could occur, producing the necessary ingredients for life in substantial quantities.
Life is governed by molecules called proteins, phospholipids and nucleotides. Past research suggests that useful molecules containing nitrogen like nitriles—cyanoacetylene(HC3N) and hydrogen cyanide (HCN)—and isonitriles—isocyanide(HNC) and methyl isocyanide(CH3NC)—could be used to make these building blocks of life. As of yet though, there has been no clear way to make all of these in the same environment in substantial amounts.