As sponges and ctenophores are such disparate animals13, the nature of the first diverging animal lineage has implications for the evolution of fundamental animal characteristics. Adult sponges are generally sessile filter-feeding organisms with body plans organized into reticulated water-filtration channels, structures built out of silica or calcium carbonate, and specialized cell types and tissues used for feeding, reproduction and self-defence, but they lack neuronal and muscle cells15. By contrast, ctenophores are gelatinous marine predators that move using eight longitudinal ‘comb rows’ of ciliary bundles16,17; they are superficially similar but unrelated to cnidarian medusae13,18 and possess multiple nerve nets19. Thus, whereas the sponge-sister scenario suggests a single origin of neurons on the ctenophore–parahoxozoan stem, the ctenophore-sister scenario implies either that either ancestral metazoan neurons were lost in the sponge lineage, or that there was convergent evolution of neurons in the ctenophore and parahoxozoan lineages3,6. Similar considerations apply to other metazoan cell types18, gene regulatory networks, animal development13,18 and other uniquely metazoan features.
Despite its importance for understanding animal evolution, the relative branching order of sponges, ctenophores and other animals has proven to be difficult to resolve2. The fossil record is largely silent on this issue as verified Precambrian sponge fossils are extremely rare20 and putative fossils of the soft-bodied ctenophores are difficult to interpret21. Morphological characters of living groups (for example, choanocytes of sponges) are not sufficient to resolve the question because true homology is difficult to assign, and such characters are easily lost or can arise convergently13,22. The ctenophore-sister hypothesis is supported by a pair of gene duplications shared by sponges, bilaterians, placozoans and cnidarians but not ctenophores23. Although sophisticated methods for sequence-based phylogenomics have been developed and applied to increasingly large molecular datasets, there is still considerable debate about the relative position of sponges and ctenophores as results are sensitive to how sequence evolution is modelled11, which taxa or sites are included24,25, and the effects of long-branch artifacts and nucleotide compositional variation26. New approaches are needed.
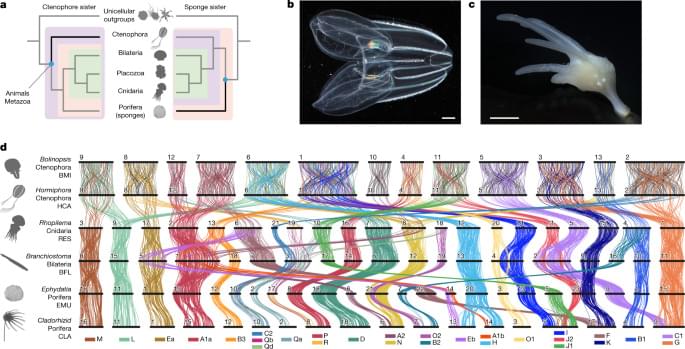
We reasoned that patterns of synteny, classically defined as chromosomal gene linkage without regard to gene order27, could provide a powerful tool for resolving the ctenophore-sister versus sponge-sister debate. Chromosomal patterns of gene linkage evolve slowly in many lineages12,28,29,30, probably because it is improbable for interchromosomal translocations to be fixed in populations with large effective population sizes28,31,32. Notably, some changes in synteny are effectively irreversible. For example, when two distinct ancestral synteny groups are combined onto a single chromosome by translocation, and subsequent intrachromosomal rearrangements mix these two groups of genes, it is very unlikely that the ancestral separated pattern will be restored by further rearrangement and fission, in the same sense that spontaneous reduction in entropy is improbable12. Such rare and irreversible changes are particularly useful for resolving challenging phylogenetic questions as they give rise to shared derived features that unambiguously unite all descendant lineages33,34,35. Deeply conserved syntenies observed between animals and their closest unicellular relatives12 suggest that outgroup comparisons could be used to infer ancestral metazoan states and polarize changes within animals to address the sponge-sister versus ctenophore-sister debate. Yet, chromosome-scale genome sequences of the unicellular or colonial eukaryotic outgroups closest to animals (choanoflagellates, filastereans and ichthyosporeans) have not been reported.