Apitherapy is an emerging field with the potential to impact the economic aspects of cancer research globally, particularly in under-resourced communities. To date, however, studies are yet to fully investigate the molecular mechanism of action of honeybee venom and melittin, and their consequent optimum usage in the oncology arena is yet to be comprehensively investigated, particularly for the treatment of breast cancer, the most commonly occurring cancer in women worldwide2. TNBCs and HER2-enriched tumors are highly aggressive breast cancer subtypes. TNBC is associated with the highest mortality and, despite frequent EGFR expression, commonly displays resistance to anti-EGFR therapies with high dependence on PI3K/Akt signaling for proliferation, survival, and chemotherapy resistance34.
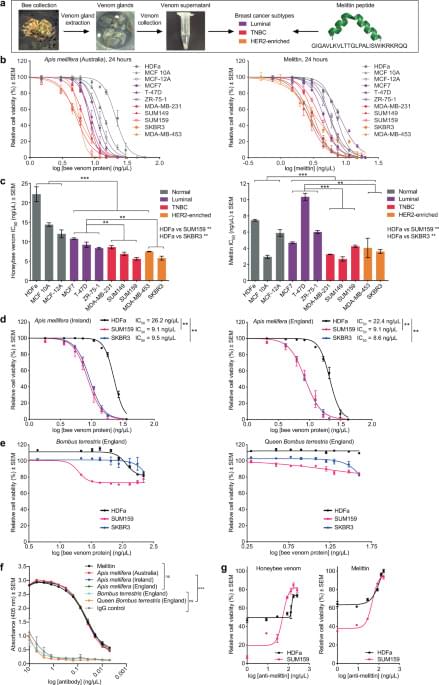
Anti-HER2 therapies have substantially improved long-term survival in early-stage HER2-positive cancers, but the majority of late-stage patients eventually develop resistance and succumb to the disease33,35,36. Not only did we demonstrate selectivity of honeybee venom and melittin for malignant cells, but we also revealed higher potencies for these aggressive types of breast cancer.
Here, we show that honeybee venom and melittin suppress the ligand-induced phosphorylation of EGFR and HER2, dynamically modulating downstream signaling pathways in breast cancer cells. We propose that melittin directly or indirectly inhibits RTK dimerization. Melittin may also enter the cell to directly or indirectly modulate downstream signaling pathways25,60. Previous work has shown that melittin can be targeted to HER2-overexpressing cell lines using immunoliposomes bearing trastuzumab61. Here, we demonstrate that melittin alone selectively targets HER2-and EGFR-overexpressing breast cancer cells. Interestingly, melittin was more potently toxic to breast cancer cells compared to honeybee venom, warranting further investigation.