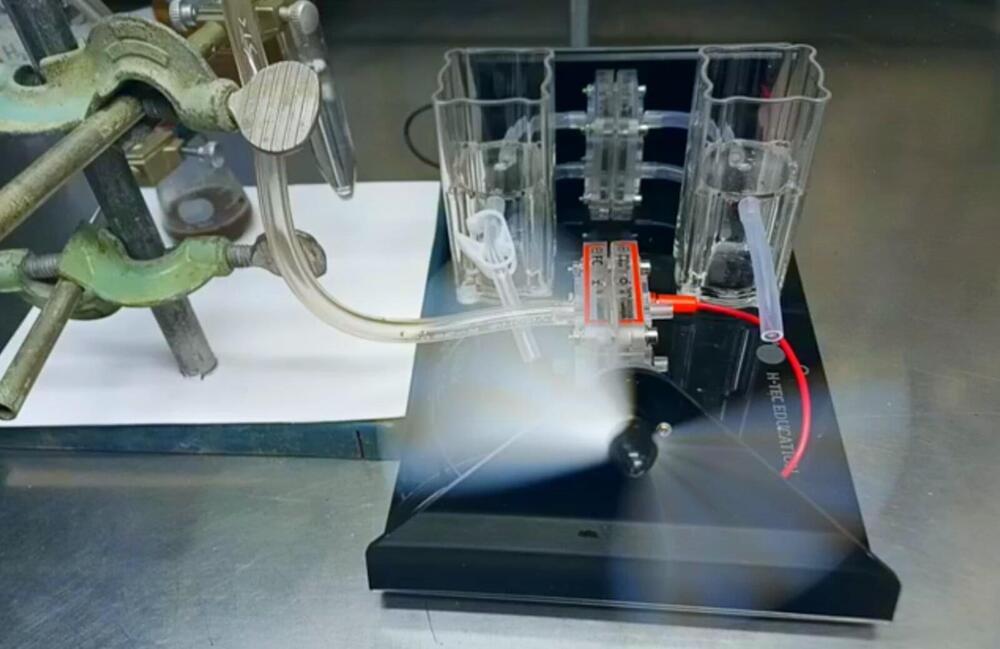
University of Alberta researchers have developed a new catalyst that could revolutionize how we generate power and purify water. When placed in any type of water and provided with a small amount of power, the catalyst produces hydrogen that can be fed into a fuel cell to generate electricity along with distilled water that is safe to drink.
The catalyst was discovered almost entirely by chance when Robin Hamilton was creating an electrode for an undergraduate student working on a waste biomass upcycling project. He mixed up a combination of powders and allowed them to sit overnight in water, intending to finish the cell the following day. When he returned in the morning, the mixture was bubbling—a reaction that was extremely out of the ordinary.
“It ends up being that when you mix these two things together, they interact, they work together and hydrogen comes off. It floored us,” says Hamilton, a senior research associate in the Department of Chemistry.