face_with_colon_three year 2012.
(Phys.org)—Both high-valued diamond and low-prized graphite consist of exactly the same carbon atoms. The subtle but nevertheless important difference between the two materials is the geometrical configuration of their building blocks, with large consequences for their properties. There is no way, any kind of matter could be diamond and graphite at the same time.
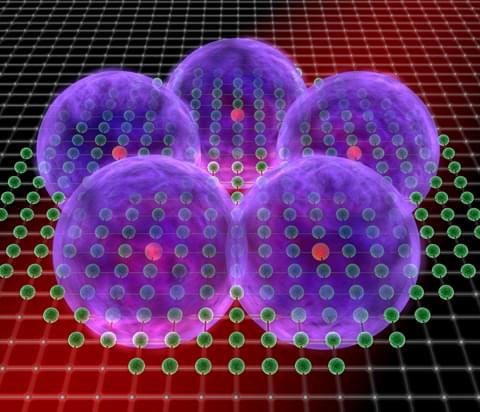
However, this limitation does not hold for quantum matter, as a team of the Quantum Many-Body Physics Division of Prof. Immanuel Bloch (Max-Planck-Institute of Quantum Optics and Ludwig-Maximilians-Universität München) was now able to demonstrate in experiments with ultracold quantum gases. Under the influence of laser beams single atoms would arrange to clear geometrical structures (Nature, November 1st, 2012). But in contrast to classical crystals all possible configurations would exist at the same time, similar to the situation of Schrödinger’s cat which is in a superposition state of both “dead” and “alive”. The observation was made after transferring the particles to a highly excited so-called Rydberg-state. “Our experiment demonstrates the potential of Rydberg gases to realise exotic states of matter, thereby laying the basis for quantum simulations of, for example, quantum magnets,” Professor Immanuel Bloch points out.