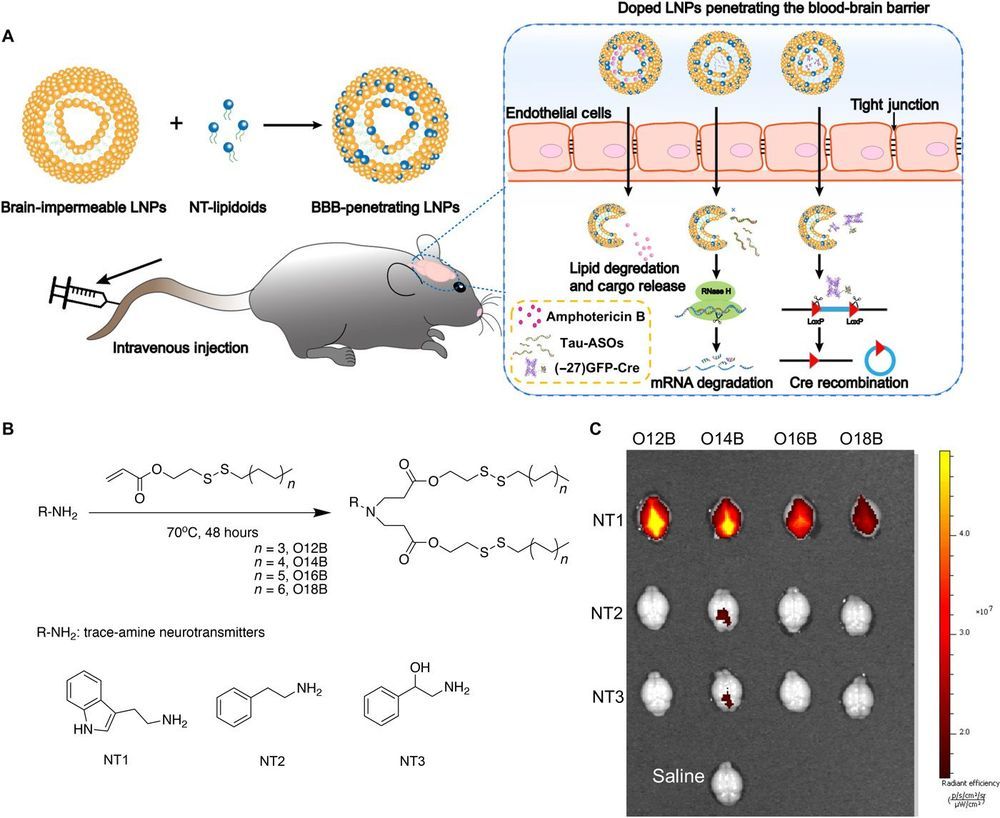
Utilizing neurotransmitters as a passport into the brain:
Safe and efficient delivery of blood-brain barrier (BBB)–impermeable cargos into the brain through intravenous injection remains a challenge. Here, we developed a previously unknown class of neurotransmitter–derived lipidoids (NT-lipidoids) as simple and effective carriers for enhanced brain delivery of several BBB-impermeable cargos. Doping the NT-lipidoids into BBB-impermeable lipid nanoparticles (LNPs) gave the LNPs the ability to cross the BBB. Using this brain delivery platform, we successfully delivered amphotericin B (AmB), antisense oligonucleotides (ASOs) against tau, and genome-editing fusion protein (−27)GFP-Cre recombinase into the mouse brain via systemic intravenous administration. We demonstrated that the NT-lipidoid formulation not only facilitates cargo crossing of the BBB, but also delivery of the cargo into neuronal cells for functional gene silencing or gene recombination. This class of brain delivery lipid formulations holds great potential in the treatment of central nervous system diseases or as a tool to study the brain function.