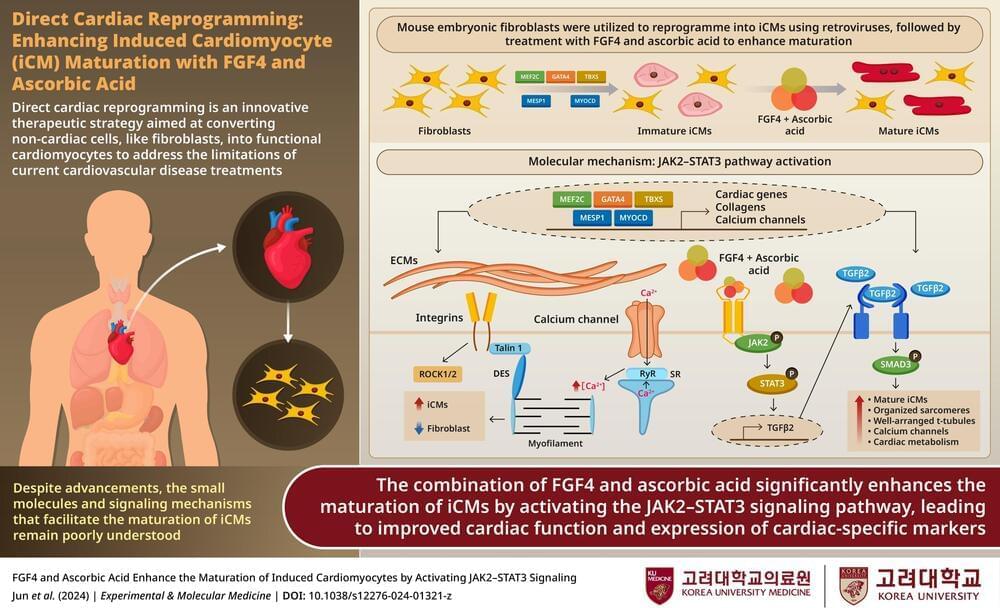
Cardiovascular disease continues to lead as the primary cause of death across the globe, taking millions of lives every year. Damage caused by these diseases is particularly difficult to repair, since the heart has minimal ability to regenerate itself. But what if we could reprogram the body’s own cells to restore damaged tissue?
This question has been tackled by scientists at Korea University, led by Dr. Myeong-Hwa Song. The team has unveiled an innovative technique to convert fibroblasts—common connective tissue cells—into mature and functional induced cardiomyocytes (iCMs). Their method relies on combining fibroblast growth factor 4 (FGF4) with vitamin C, a pairing that accelerates cell maturation and enhances function.
“Our findings bring us closer to transforming regenerative medicine into practical therapies,” says Dr. Song, who is based at Korea University’s Department of Cardiology and in Seoul, South Korea. “This research takes an important step toward using a patient’s own cells to repair their heart.”