A research team at the Institute of Materials Chemistry at TU Wien, led by Professor Dominik Eder, has developed a new synthetic approach to create durable, conductive and catalytically active hybrid framework materials for (photo)electrocatalytic water splitting. The study is published in Nature Communications.
The development of technologies for sustainable energy carriers, such as hydrogen, is essential. A promising way to produce hydrogen (H2) is from splitting water into H2 and oxygen (O2), either electrochemically or using light, or both—a path that the team follows. However, this process requires a catalyst that accelerates the reaction without being consumed. Key criteria for a catalyst include a large surface area for the adsorption and splitting of water molecules, and durability for long-term use.
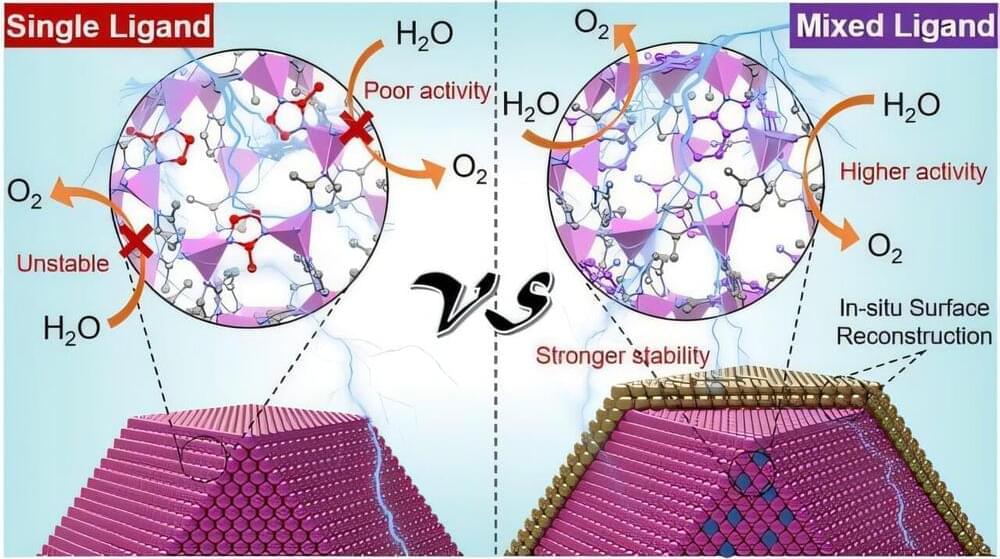
Zeolitic imidazolate frameworks (ZIFs), a class of hybrid organic/inorganic materials with molecular interfaces and numerous pores, offer record surface areas and ample adsorption sites for water as catalysts. They consist of single metal ions, such as cobalt ions, which are connected by specific organic molecules, called ligands, through what is called coordination bonds. Conventional ZIFs only contain a single type of organic ligand.








Leave a reply