Is nature really as strange as quantum theory says—or are there simpler explanations? Neutron measurements at TU Wien prove that it doesn’t work without the strange properties of quantum theory.
Can a particle be in two different places at the same time? In quantum physics, it can: Quantum theory allows objects to be in different states at the same time—or more precisely: in a superposition state, combining different observable states. But is this really the case? Perhaps the particle is actually in a very specific state, at a very specific location, but we just don’t know it?
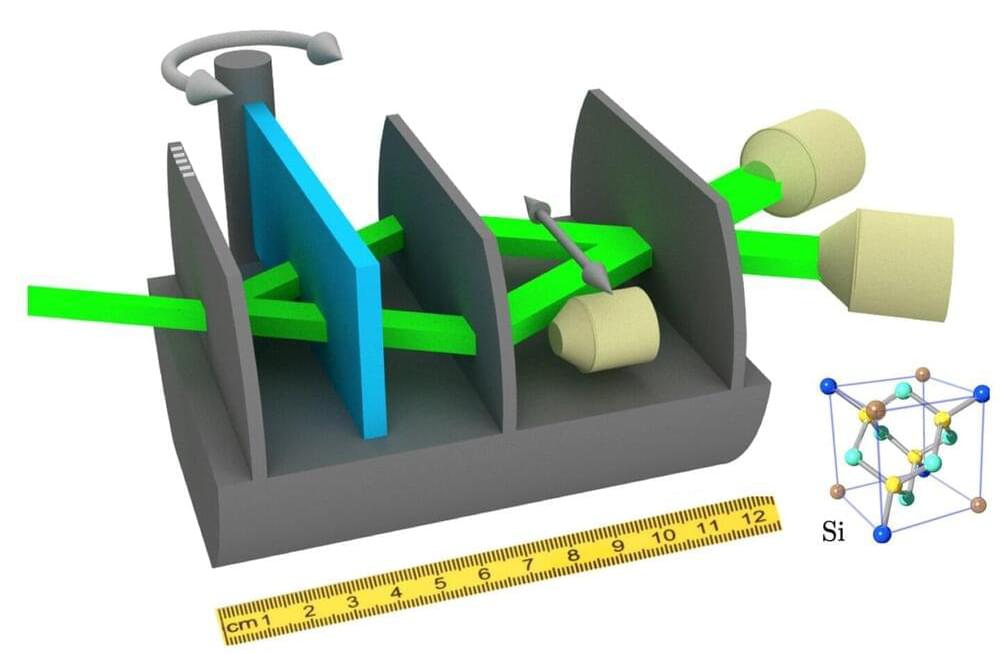
The question of whether the behavior of quantum objects could perhaps be described by a simple, more classical theory has been discussed for decades. In 1985, a way of measuring this was proposed: the so-called “Leggett-Garg inequality.” Any theory that describes our world without the strange superposition states of quantum theory must obey this inequality.








All quantum particles — nucleons including neutral particles exist in charged ultrahighdense(+) clusters with inter-répulsion forces contrasted clusters charged ultralightdensity(-) with inter-répulsion forces. Both classes exist in plasma states at ultra different densities, and as neutrons exit the positive cluster, are stripped of charge by nature of atom stability arising from maintaining equality between –ve and + ve equal charge attraction. This is the reason we cannot detach charged particles (protons) but can detach neutrons neutrally charged particles ONLY. Therefore quantum theory’s smart « vagueness » explains states, location, orientation, paths , time in the unseen undetectable probability spaces for particle theory.