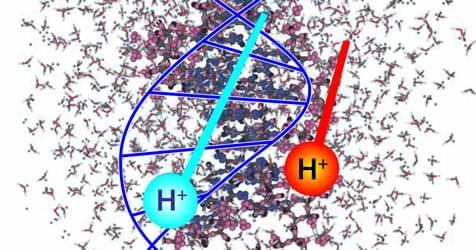
A study of the electron excitation response of DNA to proton radiation has elucidated mechanisms of damage incurred during proton radiotherapy.
Radiobiology studies on the effects of ionizing radiation on human health focus on the deoxyribonucleic acid (DNA) molecule as the primary target for deleterious outcomes. The interaction of ionizing radiation with tissue and organs can lead to localized energy deposition large enough to instigate double strand breaks in DNA, which can lead to mutations, chromosomal aberrations, and changes in gene expression. Understanding the mechanisms behind these interactions is critical for developing radiation therapies and improving radiation protection strategies. Christopher Shepard of the University of North Carolina at Chapel Hill and his colleagues now use powerful computer simulations to show exactly what part of the DNA molecule receives damaging levels of energy when exposed to charged-particle radiation (Fig. 1) [1]. Their findings could eventually help to minimize the long-term radiation effects from cancer treatments and human spaceflight.
The interaction of radiation with DNA’s electronic structure is a complex process [2, 3]. The numerical models currently used in radiobiology and clinical radiotherapy do not capture the detailed dynamics of these interactions at the atomic level. Rather, these models use geometric cross-sections to predict whether a particle of radiation, such as a photon or an ion, crossing the cell volume will transfer sufficient energy to cause a break in one or both of the DNA strands [4– 6]. The models do not describe the atomic-level interactions but simply provide the probability that some dose of radiation will cause a population of cells to lose their ability to reproduce.








Comments are closed.