Circa 2015
Coatings that attract water (hydrophilic) are useful for anti-fogging applications6; any liquid water spreads out into a thin film thereby maintaining transparency. This is more favorable than using hydrophobic surfaces for anti-fogging as this requires a surface to be tilted for the droplets to roll off and transparency be maintained. Hydrophilic surfaces can also be used for self-cleaning7. Previous examples of superhydrophilic surfaces include the use of polymer–nanoparticle coatings8,9,10,11 however mechanical durability was not investigated.
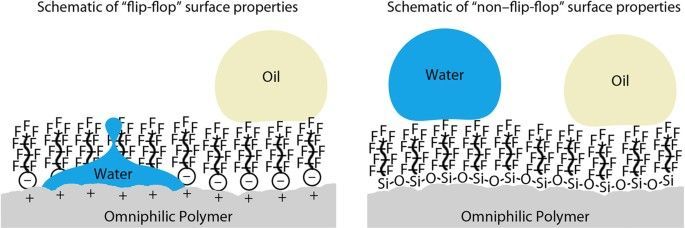
Coatings with surface tensions lower than that of water (72 mN m–1) but higher than that of oils12 (20–30 mN m–1) will attract oils (oleophilic) but repel water and can be used to create oil–water separators13,14,15. When applied to a porous substrate, the coating will allow the passage of oil but block the passage of water, resulting in their separation. In addition, their water repellency also makes them ideal for self-cleaning4,16 and anti-icing17,18,19 applications. Anti-icing surfaces are typically superhydrophobic as supercooled droplets of water are able to roll off the cold surface before freezing and any ice formed is weakly adhered compared to hydrophilic surfaces due to an air cushion18,20.
Coatings with lower surface tensions (∼ 20 mN m–1 or less) will repel both oil (oleophobic) and water and are useful for anti-fouling such as in medical and transport applications, where both the oil-repellency and nanostructuring are of importance21,22,23,24,25,26,27. Previous work was not suitable for such applications as either the durability28 or oil-repellency29 was not optimal. The oil repellency also makes these surfaces ideal for anti-smudge applications30,31 where the oils from fingers are not deposited onto the surface and the surface remains clear. The water repellency means these coatings can also be used in self-cleaning and anti-icing applications.








Comments are closed.