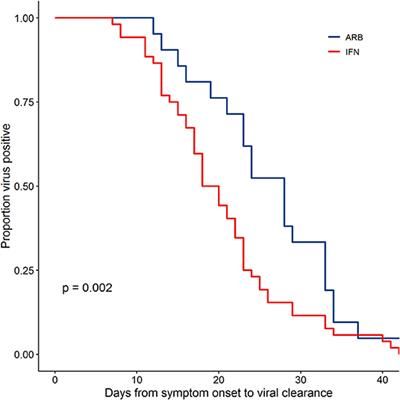
The global pandemic of COVID-19 cases caused by infection with SARS-CoV-2 is ongoing, with no approved antiviral intervention. We describe here the effects of treatment with interferon (IFN)-α2b in a cohort of confirmed COVID-19 cases in Wuhan, China. In this uncontrolled, exploratory study, 77 adults hospitalized with confirmed COVID-19 were treated with either nebulized IFN-α2b (5 mU b.i.d.), arbidol (200 mg t.i.d.) or a combination of IFN-α2b plus arbidol. Serial SARS-CoV-2 testing along with hematological measurements, including cell counts, blood biochemistry and serum cytokine levels, and temperature and blood oxygen saturation levels, were recorded for each patient during their hospital stay. Treatment with IFN-α2b with or without arbidol significantly reduced the duration of detectable virus in the upper respiratory tract and in parallel reduced duration of elevated blood levels for the inflammatory markers IL-6 and CRP. These findings suggest that IFN-α2b should be further investigated as a therapy in COVID-19 cases.
In December 2019, an outbreak of pneumonia was reported in Wuhan, Hubei province, China, resulting from infection with a novel coronavirus (CoV), severe acute respiratory syndrome (SARS)-CoV-2. SARS-CoV-2 is a novel, enveloped betacoronavirus with phylogenetic similarity to SARS-CoV (1). Unlike the coronaviruses HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU, that are pathogenic in humans and are associated with mild clinical symptoms, SARS-CoV-2 resembles both SARS-CoV and Middle East respiratory syndrome (MERS), with the potential to cause more severe disease. A critical distinction is that CoVs that infect the upper respiratory tract tend to cause a mild disease, whereas CoVs that infect both upper and lower respiratory tracts (such as SARS-CoV-2 appears to be) may cause more severe disease. Coronavirus disease (COVID)-19, the disease caused by SARS-CoV-2, has since spread around the globe as a pandemic.
In the absence of a SARS-CoV-2-specific vaccine or an approved antiviral, a number of antivirals are currently being evaluated for their therapeutic effectiveness. Type I IFNs-α/β are broad spectrum antivirals, exhibiting both direct inhibitory effects on viral replication and supporting an immune response to clear virus infection (2). During the 2003 SARS-CoV outbreak in Toronto, Canada, treatment of hospitalized SARS patients with an IFN-α, resulted in accelerated resolution of lung abnormalities (3). Arbidol (ARB) (Umifenovir) (ethyl-6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2 [(phenylthio)methyl]-indole-3-carboxylate hydrochloride monohydrate), a broad spectrum direct-acting antiviral, induces IFN production and phagocyte activation. ARB displays antiviral activity against respiratory viruses, including coronaviruses (4).









Comments are closed.