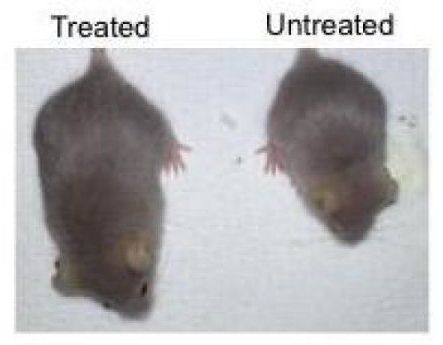
The findings, published on February 18, 2019 in the journal Nature Medicine, highlight a novel CRISPR/Cas9 genome-editing therapy that can suppress the accelerated aging observed in mice with Hutchinson-Gilford progeria syndrome, a rare genetic disorder that also afflicts humans. This treatment provides important insight into the molecular pathways involved in accelerated aging, as well as how to reduce toxic proteins via gene therapy.
“Aging is a complex process in which cells start to lose their functionality, so it is critical for us to find effective ways to study the molecular drivers of aging,” says Juan Carlos Izpisua Belmonte, a professor in Salk’s Gene Expression Laboratory and senior author of the paper. “Progeria is an ideal aging model because it allows us to devise an intervention, refine it and test it again quickly.”
With an early onset and fast progression, progeria is one of the most severe forms of a group of degenerative disorders caused by a mutation in the LMNA gene. Both mice and humans with progeria show many signs of aging, including DNA damage, cardiac dysfunction and dramatically shortened life span. The LMNA gene normally produces two similar proteins inside a cell: lamin A and lamin C. Progeria shifts the production of lamin A to progerin. Progerin is a shortened, toxic form of lamin A that accumulates with age and is exacerbated in those with progeria.









Comments are closed.