Many battery scientists are interested in the potential of lithium sulfur batteries because, at least in theory, they offer a high energy density at relatively low cost. However, lithium sulfur batteries face a number of challenges, including the low electrical conductivity of sulfur and the tendency of the cathode to expand significantly in size during the discharge cycle—a tendency that prevents the cathode material from being packed as densely in the battery as scientists would like.
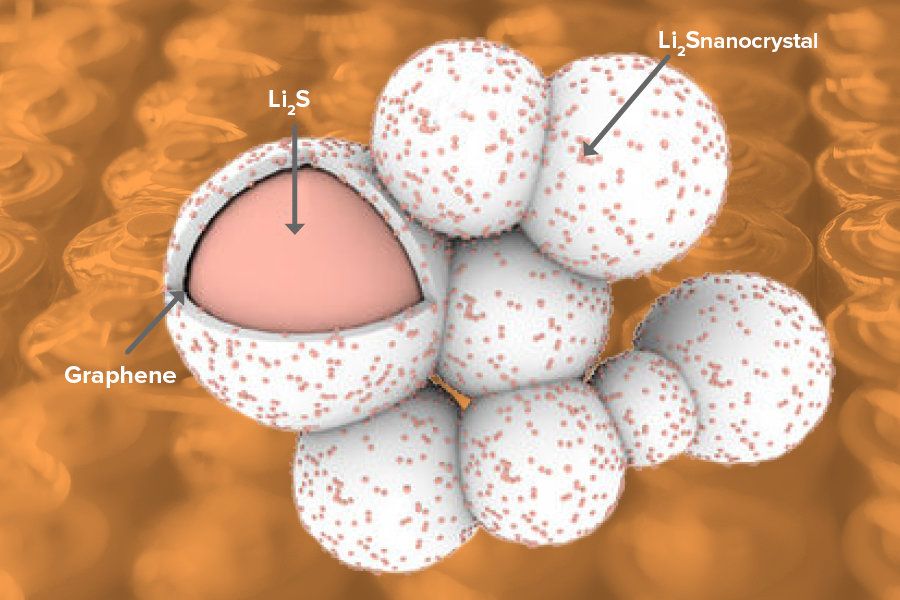
To combat these problems and bring lithium sulfur batteries closer to reality, researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory, the University of Illinois at Chicago (UIC) and Oregon State University have developed a new cathode material made of lithium sulfide that is encapsulated by graphene.
To make the cathode material, Argonne chemists Jun Lu and Khalil Amine heated lithium metal and then exposed it to carbon disulfide gas, a common industrial solvent. The creation of lithium sulfide, as well as the graphene encapsulation, happened spontaneously.








Comments are closed.