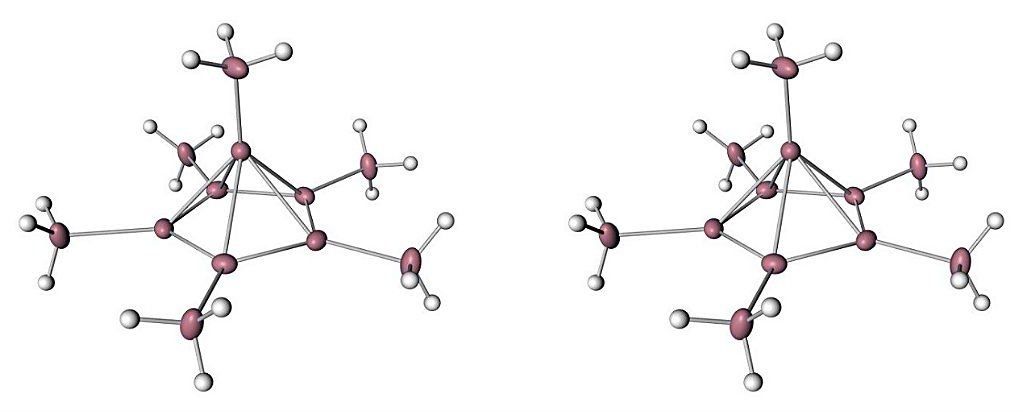
It’s the stuff of Chemistry 101: carbon can only form four bonds because it only has four shareable electrons.
But this rule no longer applies, because scientists have confirmed the existence of an exotic carbon molecule that can form six bonds, meaning the most classic example of tetravalence in our high school chemistry textbooks now comes with a hefty caveat.
If all of this is kinda giving you conniptions, we’re right there with you.
Read more








Comments are closed.