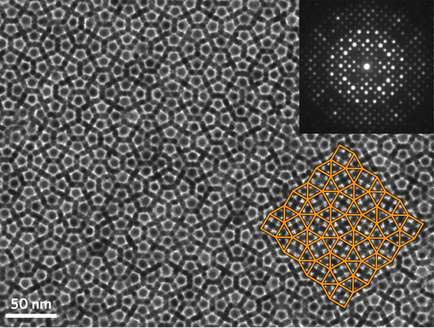
Crystals are defined by their repeating, symmetrical patterns and long-range order. Unlike amorphous materials, in which atoms are randomly packed together, the atoms in a crystal are arranged in a predictable way. Quasicrystals are an exotic exception to this rule. First discovered in 1982, their atoms pack together in an orderly fashion, but in a mosaic-like pattern that doesn’t repeat and can’t be predicted from a small sample.
Being able to map out the position of individual atoms within a quasicrystal is a prerequisite for achieving a complete understanding of their structure and aids in designing them for specific applications, but conventional microscopy techniques don’t have the resolution to accomplish such a task.
In an effort to address this challenge, researchers from the University of Pennsylvania and the University of Michigan have engineered a quasicrystal that is formed by self-assembling nanoparticles, which are an order of magnitude larger than the atoms that comprise traditional quasicrystals. Their larger size enabled the team to use a suite of microscopy and simulation techniques to deduce, for the first time, the full three-dimensional configuration of a spontaneously formed quasicrystal.








Comments are closed.