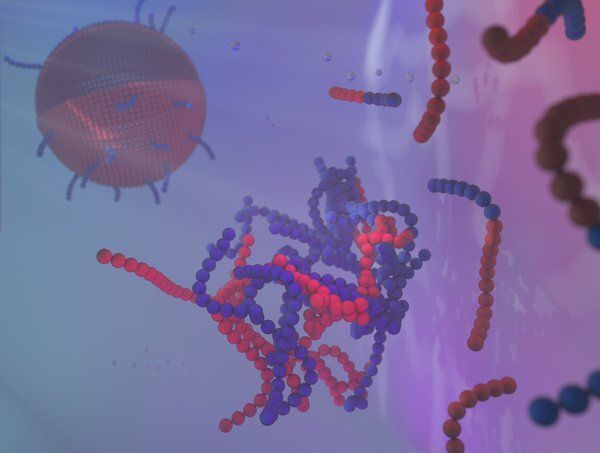
Biological membranes, and man-made variants, consist of amphiphilic molecules, of which soap is an example. These molecules have a head that bonds with water, but a tail that turns away from water. You can imagine that a group of such molecules in water, preferably puts the tails together, and sticks the heads out, towards the water. Similar processes also dominate the creation of membranes. Often they are spherical, like liposomes, so you can, for example, put a medicine in it. And also the ultimate membrane, the cell wall, is constructed in a similar way.
How nano-droplets self-assemble
Until now, the formation of ‘micelles’ was considered to be the first step in membrane formation. A micelle is an extremely small spherical structure (about 100 nanometers) of amphiphilic molecules—all with the tails inwards and the heads outwards. However, researchers at Eindhoven University of Technology discovered a different beginning: the formation of nano-droplets in water with a higher concentration of amphiphilic molecules. At the interface of that drop, the amphiphilic molecules, as it were, take each others’ hands: first they form spheres, which then change into cylinders or plates, and then a closed membrane is created that encloses the nano-droplet. With this so-called ‘self-assembly’ process, the droplet has become a liposome.
Continue reading “Nano-droplets are the key to controlling membrane formation” »