A detailed study of a reaction between a molecular ion and a neutral atom has implications for both atmospheric and interstellar chemistry.
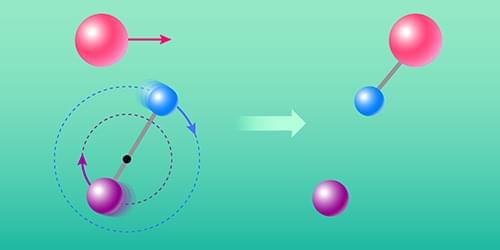
Reactions between ions and neutral atoms or molecules occur in various settings, from planetary atmospheres to plasmas. They are also the driving force behind rich reaction chains at play in the interstellar medium (ISM)—the giant clouds of gas and dust occupying the space between stars. The ISM is cold, highly dilute, and abundant with ionizing radiation [1]. These conditions are usually unfavorable for chemistry. Yet, more than 300 molecular species have been detected in the ISM to date, of which about 80% contain carbon [2]. Now Florian Grussie at the Max Planck Institute for Nuclear Physics (MPIK) in Germany and collaborators report an experimental and theoretical study of an ion–neutral reaction: that between a neutral carbon atom and a molecular ion (HD+), made of a hydrogen and a deuterium (heavy hydrogen) atom [3, 4]. The study’s findings could improve our understanding of the chemistry of the ISM.
Ion–neutral reactions are fundamentally different from those involving only neutral species. Unlike typical neutral–neutral reactions, ion–neutral reactions often do not need to overcome an activation energy barrier and proceed efficiently even if the temperature approaches absolute zero. The reason for this difference is that, in ion–neutral reactions, the ion strongly polarizes the neutral atom or molecule, causing attractive long-range interactions that bring the reactants together.