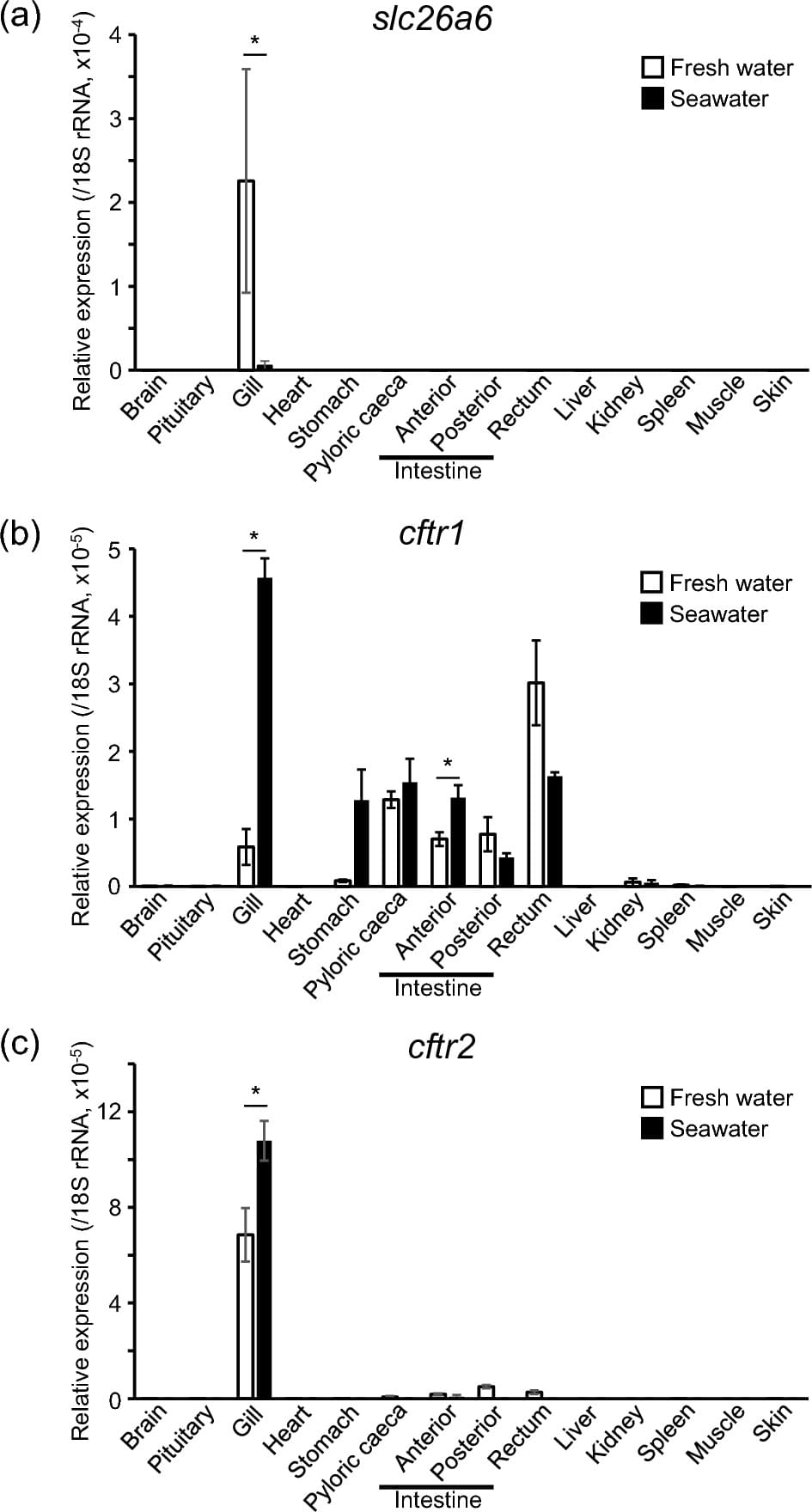
In rainbow trout, classification of ionocytes based on the expressed transporters is still in progress. The Nhe3-positive ionocytes expressing basolateral Nka and Nkcc1 and apical Nhe3b have been found in both freshwater-and seawater-acclimated rainbow trout. Colocalization of an Rh glycoprotein (Rhcg1, ammonium transporter) and Nhe3b at the apical membrane was also observed, suggesting ammonia-dependent Na+ uptake by Nhe3-positive ionocytes (Hiroi and McCormick 2012). The Nhe3-negative ionocytes, which also lack Nkcc1, are observed mainly in freshwater (Katoh et al. 2008; Hiroi and McCormick 2012). Ncc2, the apical Cl− pathway in tilapia type-II ionocytes, is thought to be absent in the gill of salmonids (Hiroi and McCormick 2012). The Nhe3-positive ionocytes showed basolateral Nka and Nkcc1 both in freshwater and seawater, suggesting that Nhe3-positive ionocytes are analogous to tilapia types-III and-IV and could be equipped with apical Cftr in seawater (Hiroi and McCormick 2012; Takei et al. 2014). However, localization of Cftr proteins by immunohistochemistry has not been successful in salmonids even with homologous antibodies (Takei et al. 2014). The mRNA of slc26a6 has been reported to be highly expressed in the gills of freshwater-acclimated rainbow trout (Boyle et al. 2015; Leguen et al. 2015) and it is very likely that this transporter is responsible for the uptake of Cl− in freshwater, but detailed localization of this protein in the gills has not been elucidated. In short, the molecules responsible for the Cl− transport across the apical membrane have not been identified in both freshwater-and seawater-acclimated rainbow trout.
Salmonids possess two cftr genes, cftr1 and cftr2 (Chen et al. 2001), and it has been reported that the expression level of both genes increases in the gill of chum salmon Oncorhynchus keta after the transfer to seawater during the juvenile stage (Wong et al. 2019). Expression of cftr1 in the gills increased also in rainbow trout after seawater transfer (Gerber et al. 2018). On the other hand, dietary salt loading reduced cftr2 expression in the gill of rainbow trout in freshwater (Kolosov and Kelly 2016). At this time, it is not clear which of these two molecules is mainly responsible for hypo-osmoregulatory Cl− secretion in the gills of salmonids.
The objective of the present study is to examine molecules responsible for the active transport of Cl− in gill ionocytes of rainbow trout. To achieve this goal, we conducted tissue distribution analyses on the expression of slc26a6, cftr1, and cftr2 in rainbow trout acclimated to freshwater or seawater. Time-course changes in the expression of these genes were also examined during seawater transfer. We localized these proteins in the gill filaments of rainbow trout acclimated to freshwater or seawater by whole-mount immunohistochemistry.