Page 10623
Dec 14, 2016
Amazon Conducts First Commercial Drone Delivery
Posted by Klaus Baldauf in category: drones
Amazon said Wednesday it made its first customer delivery by drone, putting the online retailer in the lead to use drones as a new delivery method.
Dec 14, 2016
Artificial intelligence has a big year ahead of it
Posted by Shane Hinshaw in category: robotics/AI
In 2017, AI won’t just be for the nerdy companies. Machine learning can help with mortgage applications and bridge safety, too.
Dec 14, 2016
Trump to meet tech firms including Apple, Facebook and Google
Posted by Zoltan Istvan in categories: business, employment, mobile phones, policy, robotics/AI
New article on immigration and AI in The Guardian. https://www.theguardian.com/technology/2016/dec/14/donald-tr…automation #future
All of this could be under threat if we are to take some of the comments the Trump campaign made in the run-up to the election at face value. The outspoken candidate claimed that Mark Zuckerberg’s push for specialist H1B visas (the main visa used to hire foreign talent to tech companies) was a threat to jobs for American women and minorities. Meanwhile, Trump’s chief strategist Steve Bannon suggested that Asians have too much power in Silicon Valley.
About a dozen members of Silicon Valley’s elite – including Apple CEO Tim Cook, Alphabet CEO Larry Page, Microsoft CEO Satya Nadella and Facebook COO Sheryl Sandberg – will meet with Trump in New York. The meeting is likely to provide an opportunity for them to highlight their concerns and priorities with the incoming administration.
Continue reading “Trump to meet tech firms including Apple, Facebook and Google” »
Dec 14, 2016
Can Cellular Senescence be Reversed in the Near Future, and is Reversal Desirable?
Posted by Steve Hill in categories: biotech/medical, life extension
Senescent cell removal holds great potential but are all research approaches equal?
Some scientific commentary on senescent cell clearing (Senolytics) and the different approaches the research community is engaged in.
“Researchers are taking two broad approaches to cellular senescence at the present time. The first is to build therapies that can selectively destroy senescent cells, following the SENS rejuvenation model of periodic removal of damage. If the number of senescent cells is managed so as to keep that count low, then they will not cause further harm. This has the advantage of being straightforward and requiring little further research to put into practice. A range of demonstrated treatments and potential treatments already exist — gene therapies, immunotherapies, senolytic drugs, and so forth — and companies such as Oisin Biotechnologies and UNITY Biotechnology are bringing some of these technologies to the clinic.”
Dec 14, 2016
CellAge: Senescent Cell Targeting Technology Video
Posted by Steve Hill in categories: bioengineering, biotech/medical, finance, genetics, health, life extension
Synthetic biology meets senolytics at Lifespan.io
We are developing tools to help researchers accurately target and remove dysfunctional cells in the body that have entered a state called “senescence”, and thereby assist in restoring it to youthful functionality. Please subscribe, share, and fund our campaign today! ►Campaign Link: https://www.lifespan.io/campaigns/cellage-targeting-senescen…c-biology/ ►Subscribe: https://www.youtube.com/user/LifespanIO?sub_confirmation=1
Continue reading “CellAge: Senescent Cell Targeting Technology Video” »
Dec 14, 2016
CellAge: Dr. Aubrey de Grey Endorsement Video
Posted by Steve Hill in categories: bioengineering, biotech/medical, finance, genetics, health, life extension

Dr. Aubrey de Grey from the SENS Research Foundation was kind enough to talk in support of CellAge and their campaign on Lifespan.io
We are developing tools to help researchers accurately target and remove dysfunctional cells in the body that have entered a state called “senescence”, and thereby assist in restoring it to youthful functionality. Please subscribe, share, and fund our campaign today! ►Campaign Link: https://www.lifespan.io/campaigns/cellage-targeting-senescen…c-biology/ ►Subscribe: https://www.youtube.com/user/LifespanIO?sub_confirmation=1
Continue reading “CellAge: Dr. Aubrey de Grey Endorsement Video” »
Dec 14, 2016
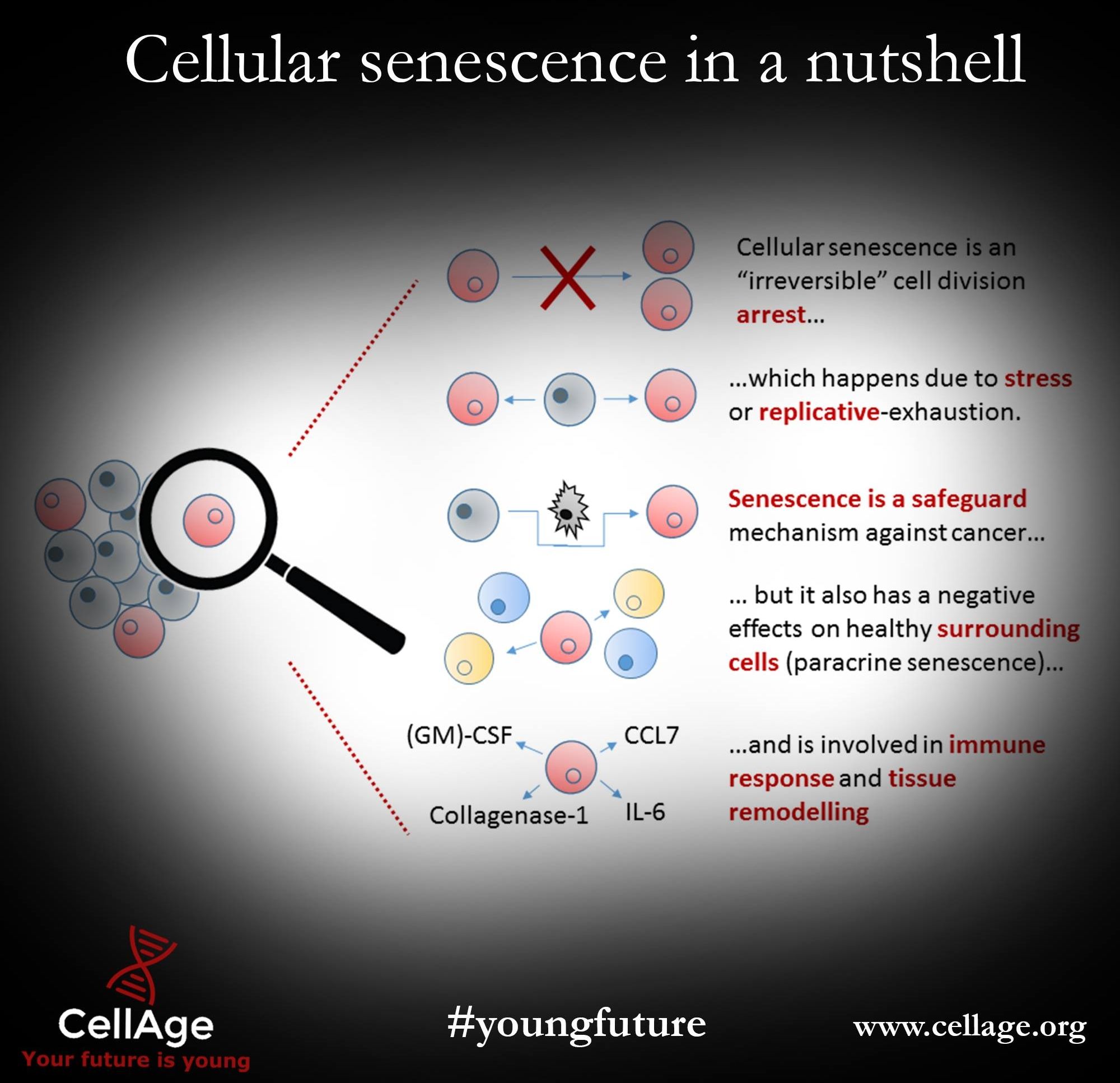
Senescent cells accumulate with age and gradually poison their neighboring cells and secrete signals that shut down your stem cells and reduce your ability to regenerate tissue
Posted by Steve Hill in categories: biotech/medical, life extension
Cellular senescence is a complicated process but here it is explained in a simple infographic. The removal of senescent cells (senolytics) is a very hot topic right now and it represents the arrival of the first of the SENS therapies.
CellAge is one of the companies engaged in senolytic research and they are running a campaign on Lifespan.io if you would like to learn more about them.
https://www.lifespan.io/campaigns/cellage-targeting-senescen…c-biology/
Dec 14, 2016
CellAge could make stem cell therapies safer
Posted by Steve Hill in categories: biotech/medical, futurism
By removing senescent cells from culture prior to transplant.
Designing synthetic promoters for safe and precise targeting of dysfunctional “senescent” cells, with the aim of developing senolytic gene therapies to remove them.
Dec 14, 2016
First approved targeted therapy for Gastric Cancer in Singapore offers new way and hope of treating disease
Posted by Karen Hurst in categories: biotech/medical, health
Lilly announced today that CYRAMZA® (ramucirumab) has been approved by the Singapore Health Sciences Authority to treat people with advanced gastric cancer, whose cancer has progressed after prior chemotherapy. First country in ASEAN to approve the new biologic therapy that extends survival in patients with advanced stomach cancer after prior chemotherapy
CYRAMZA® (ramucirumab) is now available to Singaporeans living with advanced gastric cancer. The drug gained approval by Singapore’s Health Science’s Authority (HSA) earlier this year, marking the first regulatory approval in ASEAN. CYRAMZA is already available to patients in Japan, Korea, Taiwan and Hong Kong.