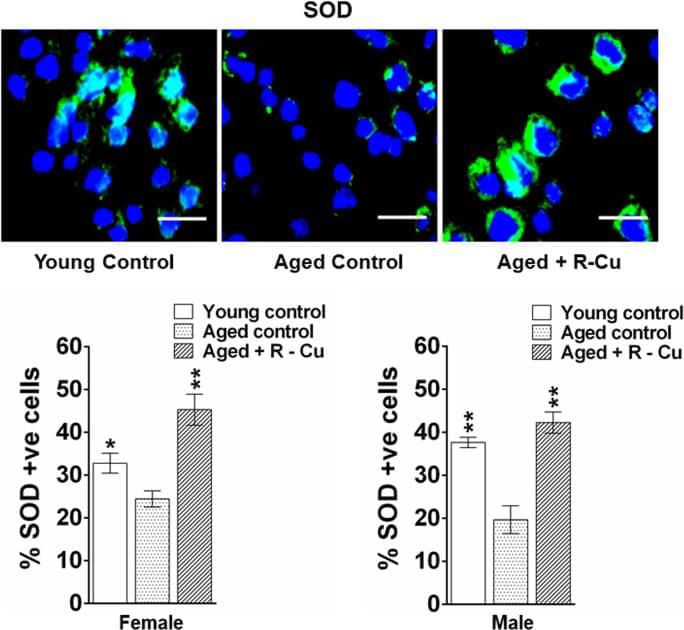
ROS are short lived molecular species containing an unpaired electron which makes them highly reactive as they search for another electron to pair with, and in the process can damage biomolecules such as DNA, proteins and lipids54. ROS induced oxidative stress is known to have multiple deleterious effects on host cells55. However, we have reported that, paradoxically, when ROS is artificially generated outside the cell in the extracellular spaces of the body, they can have wide ranging therapeutic effects18,19,20,26,27. Admixing R with Cu leads to generation of oxygen radicals by virtue of the ability of R to reduce Cu (II) to Cu (I)23,25. Oxygen radicals that are generated in the stomach upon oral administration of R–Cu are apparently absorbed to have systemic effects in the form of deactivation/eradication of extracellular cfChPs. We have shown that cfChPs have wide-ranging damaging effects on host cells. For example, cfChPs can readily enter into the healthy cells to damage their DNA, activate inflammatory cytokines and promote apoptosis via the mitochondrial pathway13,14. Given that 1 × 109–1 × 1012 cells die in the body every day56,57, we have hypothesised that repeated and lifelong assault on healthy cells by cfChPs derived from the dying cells may be the underlying cause of ageing15,16. In support of this hypothesis we show in this article that prolonged oral administration of R–Cu to ageing mice down-regulated multiple biological hallmarks of ageing and neurodegeneration by virtue of its ability to deactivate cfChPs. Our results suggest that R–Cu may qualify as an ideal anti-ageing agent since it has the potential to simultaneously retard or delay the many conditions that are associated with ageing2. To be globally applicable, an ideal anti-ageing agent should also be inexpensive and non-toxic—the two criteria that are also met by R–Cu. The latter can be easily administered orally, and both R and Cu are already approved for human use. An illustrated summary of the study design and the mechanisms by which R–Cu generated oxygen radicals eradicate cfChPs from brain micro-environment leading to down-regulation of ageing hallmarks is provided in Fig. 10.
The mechanism(s) by which R–Cu down-regulates the multiple biological hallmarks of ageing and neurodegeneration needs elaboration. Reversal of telomere shortening by R–Cu may suggest that telomere shortening could be a consequence of DNA damage inflicted by cfChPs which shear off telomere ends causing them to shorten. We observed differential effects between female and male mice with respect to telomere abnormalities. R–Cu effects in preventing telomere abnormalities in female mice were statistically significant for all parameters tested, while this was not the case in male mice. The biological explanation for this discrepant finding remains to be determined. Breakage of telomere ends may also help to explain our detection of persistent γ-H2AX signals in telomere regions of brain cells (DNA-SCARS)—an established signature of senescence43. The bare chromosomal ends can fuse with each other to lead to chromosomal instability and aneuploidy48, as was detected in our study.