Archive for the ‘energy’ category: Page 23
Jul 27, 2024
New process uses light and enzymes to create greener chemicals
Posted by Shubham Ghosh Roy in categories: chemistry, energy, food
Researchers at the Center for Advanced Bioenergy and Bioproducts Innovation (CABBI) have achieved a significant breakthrough that could lead to better—and greener—agricultural chemicals and everyday products.
Jul 27, 2024
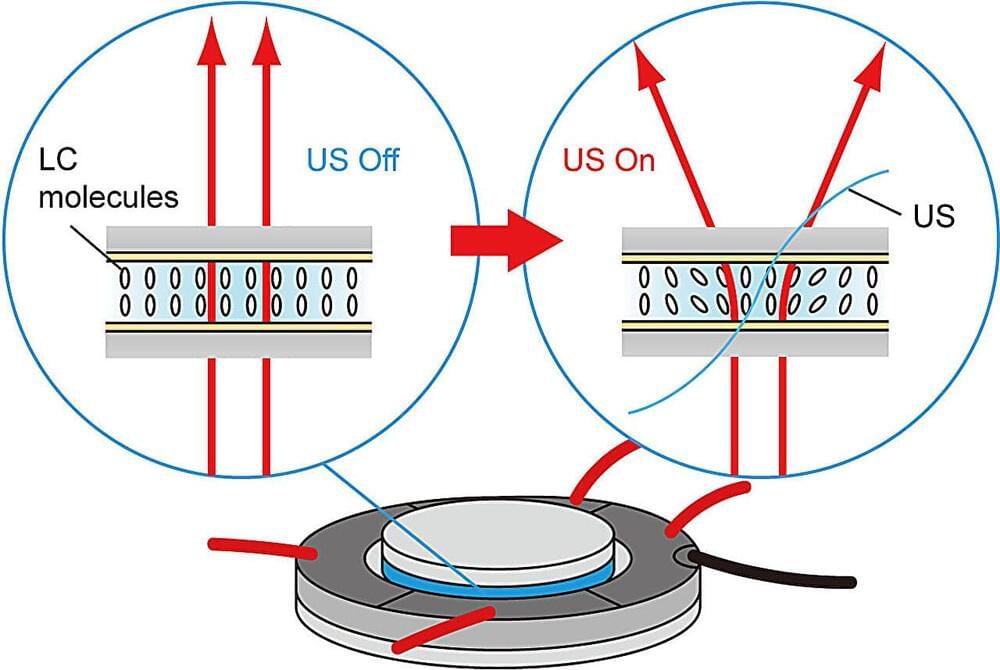
Novel tunable ultrasonic liquid crystal light diffuser paves the way for next-gen indoor lighting
Posted by Saúl Morales Rodriguéz in categories: energy, materials
This results in differences in the acoustic energy between the LC layers, glass disks, and the surrounding air, inducing an acoustic radiation force acting at the LC layer and glass disk boundary. This effect changes the molecular orientation of the LC layers, altering the transmitted light distribution. By changing the electrodes to which the input voltage is applied, the direction of the molecular orientation and therefore the diffusion directivity can be easily rotated.
The researchers investigated the diffusion characteristics of the device and found that the diffusion angle depends on the input voltage amplitude and is maximized at 16 V. Above this voltage amplitude, the diffused light can become unstable. Additionally, the transmitted light distribution depends on the polarization of incident light.
“Light diffusers that allow control over diffusion directivity can reduce energy consumption and enable users to tune the light distribution to their taste, resulting in better aesthetics Our device marks the first report of an ultrasonically controllable optical diffuser based on LC material, providing users control over diffusion directivity within a small space,” said Prof. Koyama.
After hitting a power-output milestone, fusion technology is ready to graduate from small-scale lab experiment to full-sized power plant.
Jul 26, 2024
The Development of Transhumanism in China — Article by Peter Wang
Posted by Dan Breeden in categories: economics, education, energy, food, policy, transhumanism
Ancient Chinese society was dominated by feudalism. The economy was dominated by agriculture, and the development of science and technology was slow or even suppressed. The main achievements of this era were the four major inventions of China: papermaking, gunpowder, the compass, and printing. Why was this so? For an ancient civilization with a history of several thousand years, why was the development of science and technology so backward? The fundamental reason was the idea of imperial power. Ancient China was centered on the emperor, and everything on the Chinese land was owned by the emperor, including the farmers on that land. The emperor was afraid of a peasant revolution and was afraid that others would take the emperor’s place, and as a result successive emperors would use the policy of fools. Instead of allowing farmers to read books, the emperors just wanted the farmers to plant the land every day, like slaves, so that the farmers would have no ability to overthrow the rulers. This idea of imperial power had greatly suppressed the development of science and technology.
In 1949, Mao Zedong established the first democratic, self-improving, unified China in Chinese history: The People’s Republic of China, a stable country; a country without feudal ideas; and a country that serves the people. Only then did China begin to truly develop its own education, technology, and industry. It was aimed for ordinary people to have food to eat, houses to live in, and books to read, and it was also intended for them to be more involved in technology and democracy. However, Chinese politics had hindered the development of science and technology (superhuman science), such as the Great Leap Forward, which severely reduced China’s productivity and starved many people; the Cultural Revolution had destroyed China’s economic development, education, and technology, bringing China back to pre-liberation overnight. These events were relatively unfortunate. Political struggles have severely hindered the development of science and technology (superhuman science) in China.
In 1978, China began reform and opening up. This phase of reform and opening up was China’s greatest era. China has changed from a closed country to an open country. Deng Xiaoping formulated a basic national policy centered on economic construction, which has enabled China’s economy to develop rapidly. At this time, China attaches great importance to the development of education, science and technology, and the economy. At the same time, special attention is also paid to foreign exchanges, and advanced education and technology have been introduced from abroad. In education, a large number of international students are sent to study in developed countries such as the United States, which has cultivated a large number of scientific and technological talents for China; economically, a large number of foreign companies have been introduced to optimize state-owned enterprises and support for private enterprises, so China’s economy has developed rapidly.
Jul 26, 2024
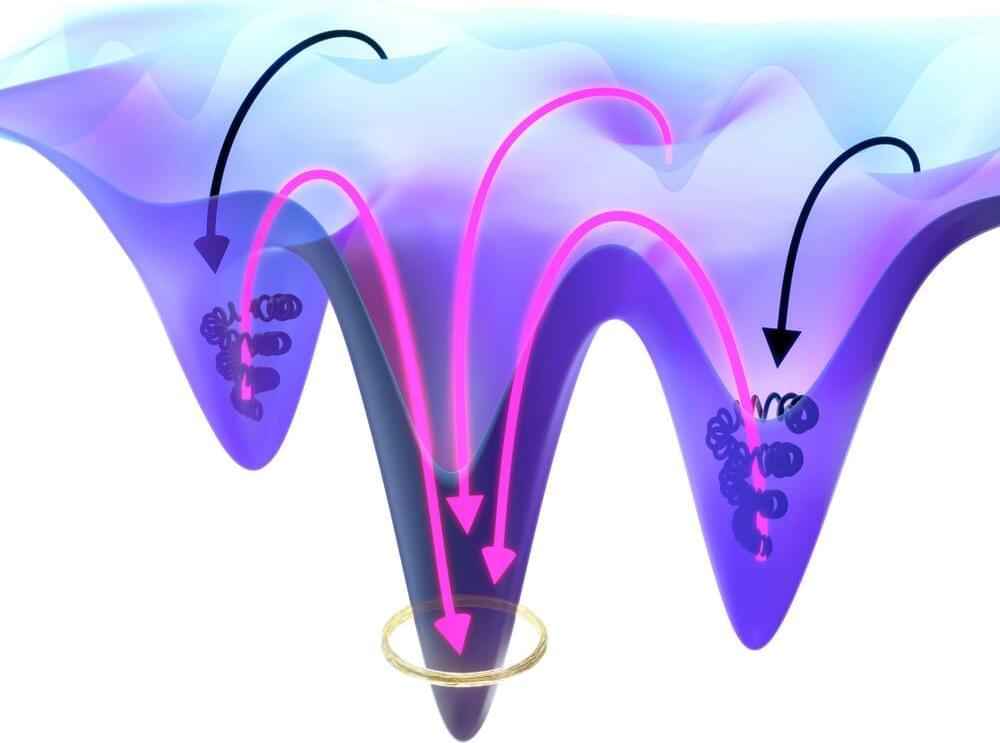
Escaping kinetic traps: How molecular interactions make it possible to overcome the energy barrier
Posted by Saúl Morales Rodriguéz in categories: energy, nanotechnology, physics
In a paper in Physical Review Letters scientists from the department Living Matter Physics at the Max Planck Institute for Dynamics and Self-Organization (MPI-DS) propose a mechanism on how energy barriers in complex systems can be overcome. These findings can help to engineer molecular machines and to understand the self-organization of active matter.
Jul 25, 2024
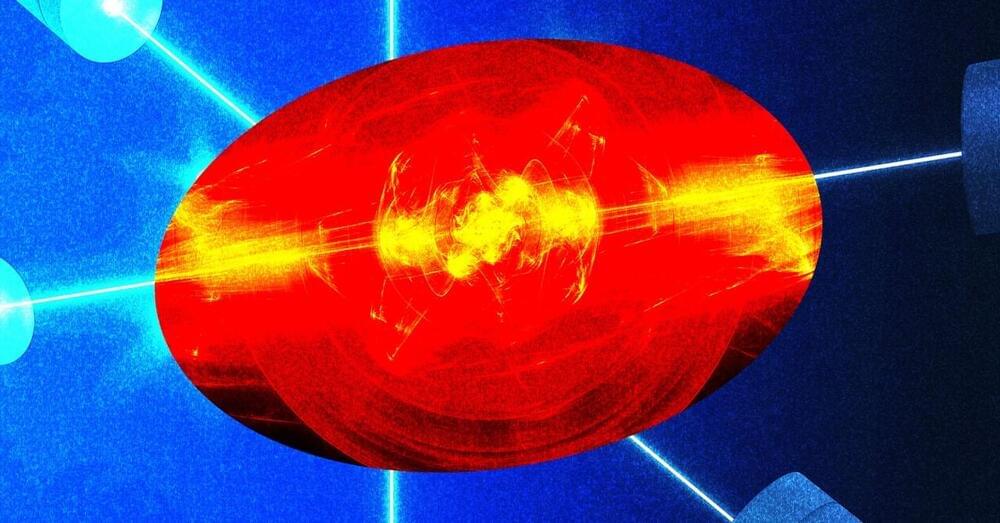
LLNL researchers uncover key to resolving long-standing ICF hohlraum drive deficit
Posted by Shubham Ghosh Roy in category: energy
In inertial confinement fusion experiments, lasers at Lawrence Livermore National Laboratory’s National Ignition Facility focus on a tiny fuel capsule suspended inside a cylindrical x-ray oven called a hohlraum. (Photo: Jason Laurea)
Jul 25, 2024
Sustainable Million-Mile Battery Bet Pays Off For Mercedes-Benz
Posted by Genevieve Klien in categories: energy, finance, sustainability, transportation

“The technological focus is on significant increases in range through advances in energy density and the reduction of charging times,” Mercedes explained, noting that the partnership cements a reliable EV battery cell supply chain while providing financial support for Farasis to build a factory in Germany.
How Sustainable Is A Million-Mile EV Battery?
Continue reading “Sustainable Million-Mile Battery Bet Pays Off For Mercedes-Benz” »
Jul 25, 2024
Toyota Eyes Perovskite Solar Cells For Solar Car Of The Future
Posted by Quinn Sena in categories: energy, space, sustainability

Fans of perovskite solar cell technology have been promising the moon, and stakeholders are increasingly confident that it will deliver. Among them is Toyota, which has just tapped its Woven Capital branch to put down a 5.5 billion yen stake in the perovskite solar startup EneCoat Technologies. If you’re thinking the solar-powered electric car of the future is coming, that’s a good guess, because EneCoat lists mobility applications among its areas of focus.
New Solar Cells For The Solar Car Of The Future
Continue reading “Toyota Eyes Perovskite Solar Cells For Solar Car Of The Future” »
While obtaining H2 from water splitting offers a promising strategy for renewable fuel production, current technologies rely on liquid freshwater. Here, authors use a hygroscopic electrolyte to achieve electrocatalytic water vapor splitting driven by renewable resources without liquid water.