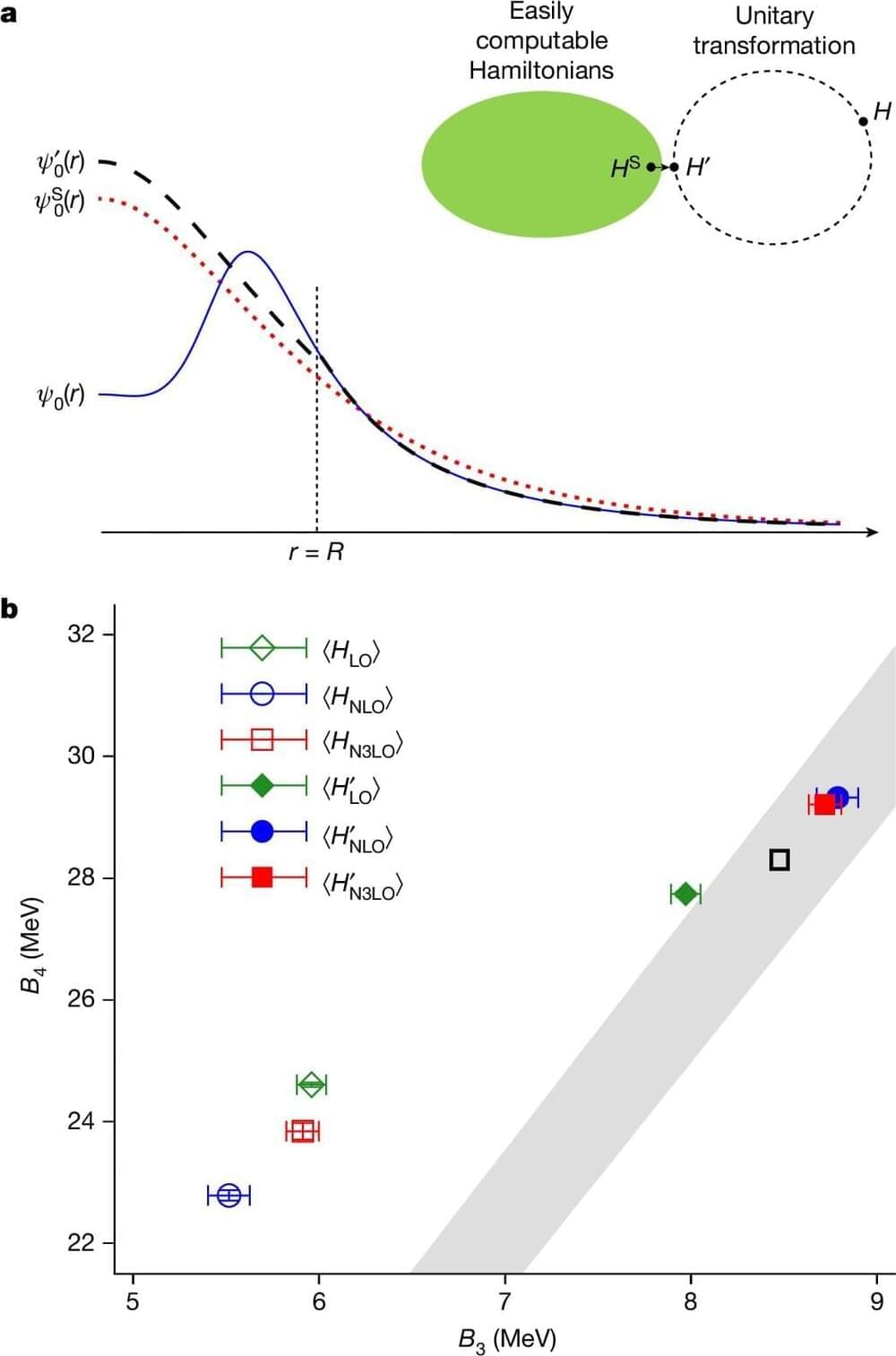
Strongly interacting systems play an important role in quantum physics and quantum chemistry. Stochastic methods such as Monte Carlo simulations are a proven method for investigating such systems. However, these methods reach their limits when so-called sign oscillations occur. This problem has now been solved by an international team of researchers from Germany, Turkey, the USA, China, South Korea and France using the new method of wavefunction matching. As an example, the masses and radii of all nuclei up to mass number 50 were calculated using this method. The results agree with the measurements, the researchers now report in the journal “Nature.”
All matter on Earth consists of tiny particles known as atoms. Each atom contains even smaller particles: protons, neutrons and electrons. Each of these particles follows the rules of quantum mechanics. Quantum mechanics forms the basis of quantum many-body theory, which describes systems with many particles, such as atomic nuclei.
One class of methods used by nuclear physicists to study atomic nuclei is the ab initio approach. It describes complex systems by starting from a description of their elementary components and their interactions. In the case of nuclear physics, the elementary components are protons and neutrons. Some key questions that ab initio calculations can help answer are the binding energies and properties of atomic nuclei and the link between nuclear structure and the underlying interactions between protons and neutrons.