Nov 19, 2020
Near-infrared probe decodes telomere dynamics
Posted by Kevin Huang in categories: biotech/medical, chemistry, life extension
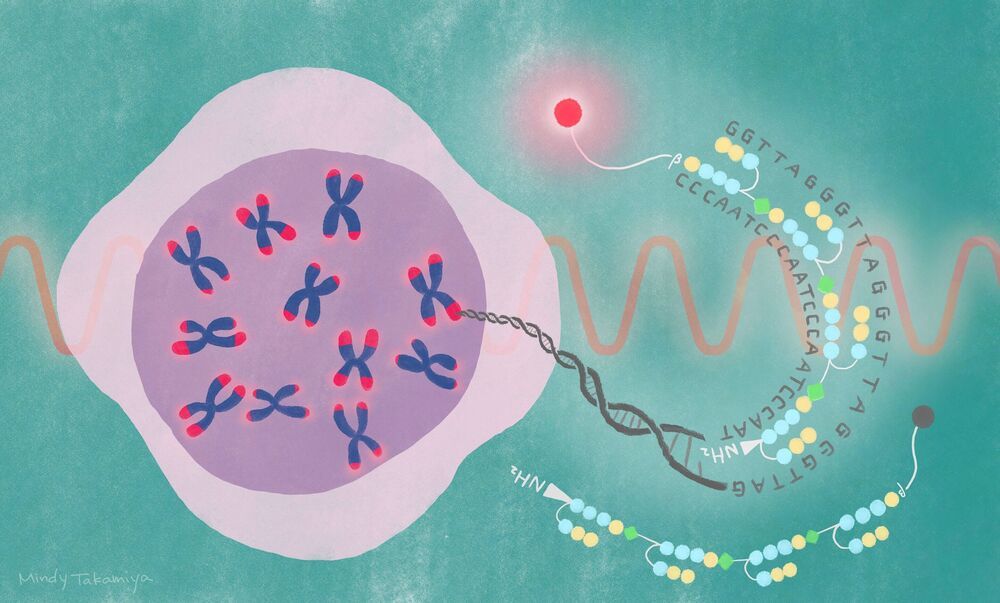
A new synthetic probe offers a safe and straightforward approach for visualizing chromosome tips in living cells. The probe was designed by scientists at the Institute for Integrated Cell-Material Science (iCeMS) and colleagues at Kyoto University, and could advance research into aging and a wide range of diseases, including cancers. The details were published in the Journal of the American Chemical Society.
“Chromosome ends are constantly at risk of degradation and fusion, so they are protected by structures called telomeres, which are made of long repeating DNA sequences and bound proteins,” says iCeMS chemical biologist Hiroshi Sugiyama, who led the study. “If telomeres malfunction, they are unable to maintain chromosome stability, which can lead to diseases such as cancer. Also, telomeres normally shorten with each cell division until they reach their limit, causing cell death.”
Visualizing telomeres, especially their physical arrangements in real-time, is important for understanding their relevance to disease and aging. Several visualization approaches already exist, but they have disadvantages. For example, some can only observe telomeres in preserved, or fixed, cells. Others are time-consuming or involve harsh treatments that denature DNA.